Archive for December 2019
FDA Approves Diffusion-Weighted Images for Elekta Unity, Expanding Options for Assessment During Therapy
Elekta today announced that it has received 510(k) premarket notification from the U.S. Food and Drug Administration for the use of diffusion-weighted MR images (DWI) obtained with Elekta Unity to be interpreted by a trained physician. This expands the clinical utility of Elekta Unity to include biologic assessment of tumor response during therapy, allowing treatment…
Read MoreFirst Patient Enrolls in New Trial for Abiomed’s Impella Looks for Heart Failure Reduction
Abiomed (NASDAQ: ABMD) announces initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial (RCT), which will explore whether unloading the heart’s left ventricle for 30 minutes with an Impella heart pump prior to opening blocked arteries will reduce infarct size after a heart attack and lead to a reduction in future heart failure rates.…
Read MoreAptar’s Nasal Unidose Device Approved by U.S. FDA for First Nasal Rescue Treatment for Frequent Seizure Activity
AptarGroup, Inc., a global leader in drug delivery, consumer product dispensing and active packaging solutions, today announced that its patented Unidose Liquid System is the device delivering the first and only nasal rescue treatment approved by the U.S. FDA, which has recently launched in the U.S. to treat acute repetitive seizures in people living with…
Read MoreImpediMed Receives FDA 510(k) Clearance of SOZO for Expanded Indication
ImpediMed Limited (ASX.IPD), a medical software technology company that non-invasively measures, monitors and manages fluid status and tissue composition using bioimpedance spectroscopy (BIS), recently announced the issuance of a further 510(k) clearance for SOZO® by the U.S. Food and Drug Administration (FDA). SOZO is the first device to gain FDA clearance for use as an…
Read MoreBeta Bionics Receives FDA Breakthrough Device Designation for the iLet Bionic Pancreas System
Beta Bionics, Inc. — a medical technology company developing and aiming to commercialize the world’s first fully automated bionic pancreas — today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its investigational iLet Bionic Pancreas System. The iLet Bionic Pancreas System is a pocket-sized, wearable investigational medical…
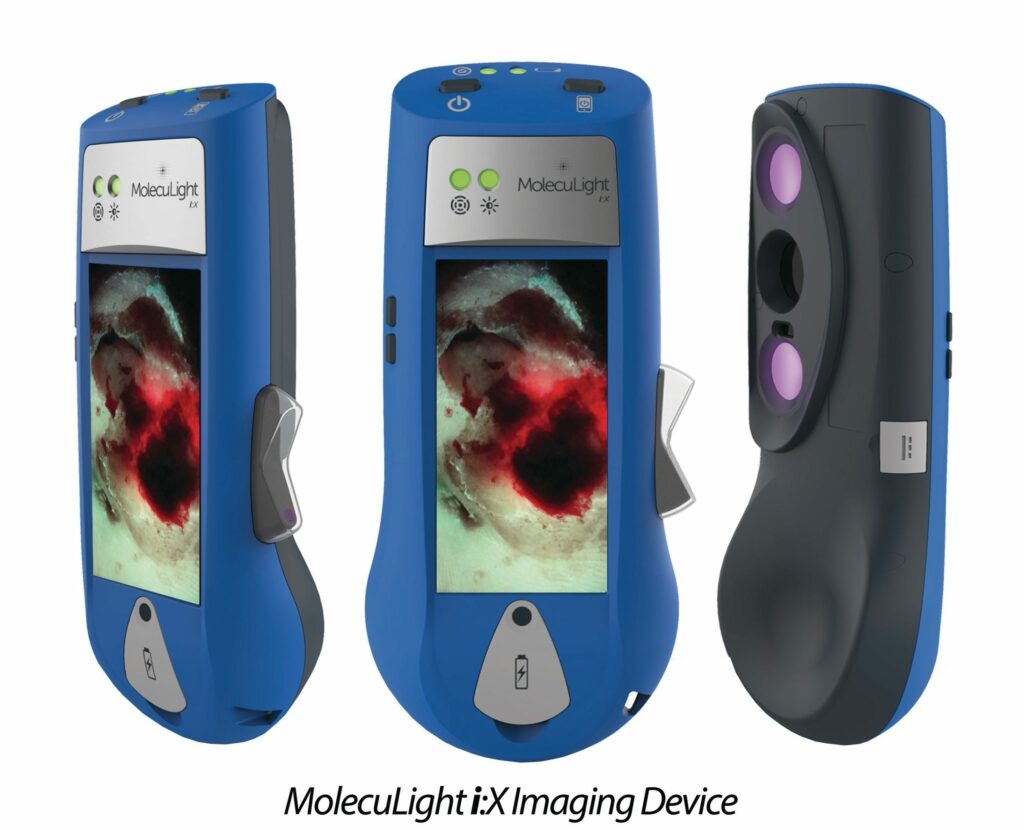
Read MoreMolecuLight Receives FDA 510(k) Clearance for its i:X Handheld Fluorescence Imaging Device for Wound Management
MolecuLight Inc., the world’s leader in handheld fluorescence imaging for real-time visualization of fluorescence in wounds, has received FDA 510(k) clearance for its i:X® handheld fluorescence imaging device for use in the detection of wounds containing bacteria. This FDA 510(k) clearance is an expansion of the original de novo clearance for the MolecuLight i:X platform, granted on August 14, 2018. The…
Read MoreNihon Kohden Launches Life Scope SVM-7200 Series Vital Sign Monitor, a Portable, Easy-to-Use Monitor with Customizable Early Warning Scoring
Nihon Kohden, a U.S. market leader in precision medical products and services, today announced the launch of its Life Scope® SVM-7200 Series vital signs monitor, a monitor designed for outpatient facilities and beds that traditionally are not continuously monitored. The monitor allows healthcare practitioners to quickly and easily measure three vital signs – blood oxygen,…
Read MoreIRRAflow Wins CONNECT’s “2019 Most Innovative New Product” Award
Breakthrough medical device that transforms treatment of intracranial bleeding recognized as most innovative new medical device from southern California. IRRAS AB, a global healthcare company with a comprehensive portfolio of innovative products for neurocritical care, announced today that IRRAflow has been selected as the 2019 winner of CONNECT’s Most Innovative New Product award in the medical…
Read MoreVicarious Surgical Wins FDA Breakthrough Designation for Surgical Robot
Vicarious Surgical Inc., the leading innovator in virtual reality and robotics for minimally invasive surgical treatments, announced today they were recently granted the Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA). The FDA Breakthrough Devices Program is intended to help patients receive more timely access to breakthrough technologies that have the potential…
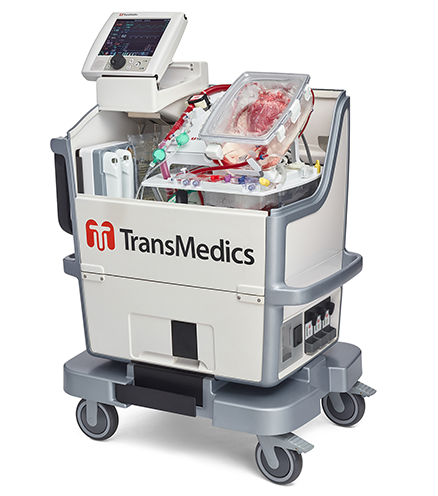
Read MoreDoctors ‘reanimate’ heart for first-of-its-kind transplant in the US
TransMedics announced today that the doctors at two hospitals performed the first U.S. heart transplants using donor hearts that were resuscitated using the company’s organ care system (OCS). The transplants from donation after circulatory death (DCD) took place at Duke University Hospital in Durham, N.C. and Massachusetts General Hospital in Boston. In a DCD procedure, the…
Read More