Archive for September 2019
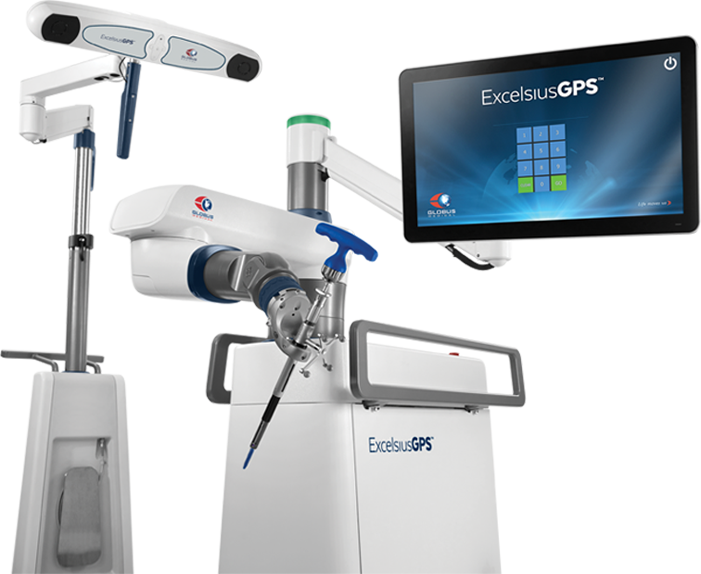
Globus Medical Acquires StelKast, Develops Orthopedic Surgery Robotics
Globus Medical Inc. last week acquired most of the assets of privately held orthopedics manufacturer StelKast Inc. for $24.1 million. The purchase price could increase by $4.3 million if certain product and sales milestones are met, including a system for robot-assisted joint reconstruction expected to launch late next year, said the companies. Audobon, Penn.-based Globus Medical makes…
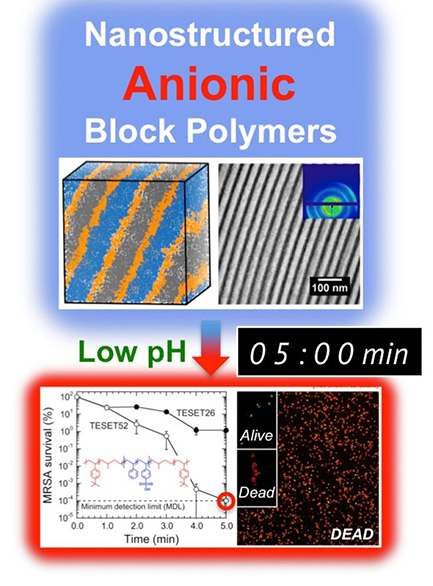
Read MoreSelf-Sterilizing Polymer Proves Effective Against Drug-Resistant Pathogens
Researchers from North Carolina State University have found that an elastic polymer possesses broad-spectrum antimicrobial properties, allowing it to kill a range of viruses and drug-resistant bacteria in just minutes – including methicillin-resistant Staphylococcus aureus (MRSA). “We were exploring a different approach for creating antimicrobial materials when we observed some interesting behavior from this polymer and decided…
Read MoreViseon, Inc. Announces US FDA Clearance and Clinical Use of its Advanced High-Definition Real Time Imaging Technology for Minimally Invasive Spine Surgery
Viseon, Inc. today announced US FDA clearance and initial clinical use of their Voyant System, integrating minimally invasive surgical (MIS) access with real time high definition imaging technology for more efficient procedural workflow, which enhances a clinician’s intraoperative visualization, providing a more accurate, precise and unimpeded view of the operative field. The Viseon technology offers…
Read MoreBiotronik First to Land MDR Certification for a High-Risk Device
Biotronik said that it is the first medtech manufacturer to receive European Medical Device Regulation (MDR) certification for a Class III (highest risk) medical device. The newly certified device is Biotronik’s Renamic programmer software, which enables physicians to program and test implanted cardiac devices such as pacemakers, implantable cardioverter-defibrillators and cardiac resynchronization therapy systems, according to…
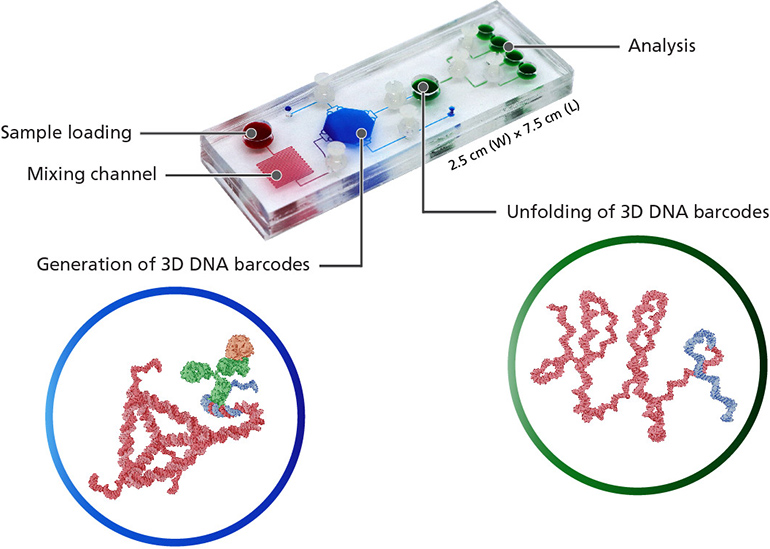
Read MoreMinimally Invasive Biopsies Provide Maximum Pathology Data
Current pathology techniques for analyzing biopsy tissues are lacking in their ability to detect cancer in small samples. Being able to rapidly study the distribution of protein expression within cells, gathered from minuscule samples, could be an important tool for early diagnosis and monitoring of cancer. Now, researchers at National University of Singapore have reported…
Read MoreSaranas Launches Early Bird® Bleed Monitoring System in the U.S.
Saranas, Inc. announced the commercial launch of the Early Bird Bleed Monitoring System in the United States. The Early Bird is the first and only device for the monitoring and early detection of endovascular bleed complications through a novel application of bioimpedance sensors. Saranas will demonstrate the device in Booth 1755 at the Transcatheter Cardiovascular Therapeutics (TCT) Conference…
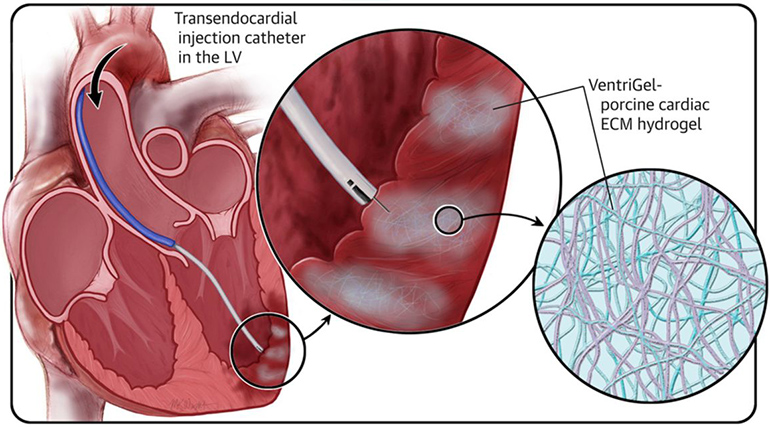
Read MoreFDA Phase 1 Trial Shows Hydrogel to Repair Heart Is Safe to Inject in Humans—A First
Ventrix, a University of California San Diego spin-off company, has successfully conducted a first-in-human, FDA-approved Phase 1 clinical trial of an injectable hydrogel that aims to repair damage and restore cardiac function in heart failure patients who previously suffered a heart attack. The trial is the first to test a hydrogel designed to repair cardiac…
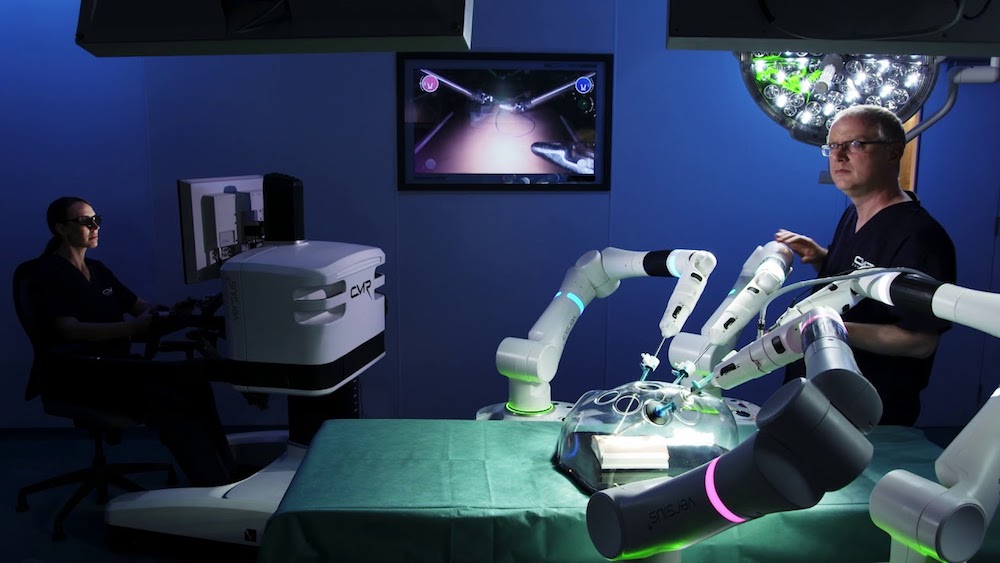
Read MoreCMR Surgical Raises $240M for Versius Surgical Robot
Cambridge, U.K.-based CMR Surgical raised a $240 million Series C round for its Versius surgical robot. CMR Surgical said it will use the funding to launch the Versius surgical robot “imminently” in Europe and Asia, with “further international expansion expected thereafter.” The Series C round included existing backers LGT Lightstone, Escala Capital Investments, Cambridge Innovation Capital, Watrium,…
Read MoreAbbott Wins CE Mark for Pediatric Heart Devices
Abbott said today that it won CE Mark approval for its Masters HP mechanical heart valve and Amplatzer Piccolo occluder. The Masters HP is the world’s smallest mechanical heart valve (15mm) and is designed for implantation in the mitral or aortic position to mimic a healthy heart valve by opening and closing to facilitate blood…
Read MoreFDA Clears ARTIS icono Family of Angiography Systems From Siemens Healthineers
The U.S. Food and Drug Administration (FDA) has cleared the ARTIS icono, a high-precision family of angiography systems from Siemens Healthineers that permit a wide range of minimally invasive procedures to be performed in a single interventional suite. The ARTIS icono biplane system is engineered for optimal utilization in neuroradiology and abdominal imaging, while the…
Read More