Archive for September 2019
FDA Clarifies Refuse-to-Accept Policy for 510(k) Applications
The FDA has updated its final guidance on its “refuse-to-accept” policy for 510(k) submissions. The guidance includes detailed checklists for devices that applicants want to be considered for traditional, abbreviated or special 510(k) clearance. The agency said it will respond to applications within 15 calendar days from submission and will notify applicants if information is missing. When…
Read MoreUltrasound Sensor Aids Diagnosis of Middle-ear Infection
A new type of ultrasound transducer from Fraunhofer should soon be delivering a fast and reliable diagnosis of infection of the middle ear. A U.S. company and the Fraunhofer Institute for Photonic Microsystems IPMS are collaborating on the development and application of this technology. The transducer is integrated in an otoscope and helps physicians decide…
Read MoreCochlear and GN Hearing Become World’s First to Support Direct Android Streaming to Hearing Devices Using Bluetooth Low Energy
Collaboration with Google on hearing aid specification now officially brings direct streaming of music, phone calls and other sound to people with hearing loss. For the first time, people can stream sound from their compatible Android devices to their hearing devices using Bluetooth Low Energy. GN Hearing, the global leader in hearing aid connectivity, and…
Read MoreGE Healthcare Receives FDA Clearance of First Artificial Intelligence Algorithms Embedded On-Device to Prioritize Critical Chest X-ray Review
GE Healthcare today announced the Food and Drug Administration’s 510(k) clearance of Critical Care Suite, an industry-first collection of artificial intelligence (AI) algorithms embedded on a mobile X-ray device. Built in collaboration with UC San Francisco (UCSF), using GE Healthcare’s Edison platform, the AI algorithms help to reduce the turn-around time it can take for…
Read MoreFDA Grants EBR Systems Breakthrough Device Designation Status for the WiSE Cardiac Resynchronization Therapy (CRT) System
EBR Systems, Inc., developer of the world’s only wireless cardiac pacing system for heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the WiSE CRT System for the treatment of heart failure. The FDA created this designation and its associated program in 2017 for certain devices…
Read MoreBaxter Announces Acquisition of Cheetah Medical to Expand Specialized Patient Monitoring Portfolio
Baxter International Inc., a leading global medical products company, today entered into a definitive agreement to acquire Cheetah Medical, a leading provider of non-invasive hemodynamic monitoring technologies. The agreement demonstrates Baxter’s ongoing commitment to improving clinical outcomes with an established patient monitoring technology to better inform and guide clinicians’ treatment decisions. Cheetah Medical is a…
Read MoreTivus Ultrasound System for Pulmonary Arterial Hypertension Gets FDA Breakthrough Designation
SoniVie, an Israeli company developing a novel system for the treatment of PAH, today announced that it has been granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the Therapeutic Intra-Vascular Ultrasound (TIVUS) System in patients with PAH. “PAH is classified as a life-threatening or irreversibly debilitating disease because it is…
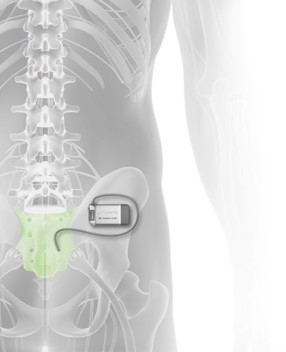
Read MoreAxonics® Announces U.S. Food & Drug Administration Approval for its Sacral Neuromodulation System
Axonics Modulation Technologies, Inc. (NASDAQ: AXNX), a medical technology company that has developed and is commercializing a novel implantable rechargeable sacral neuromodulation (“SNM”) device for the treatment of urinary and bowel dysfunction, today announced the approval of the Axonics r-SNM® System by the United States Food & Drug Administration (“FDA”). The Axonics System is the first rechargeable SNM system…
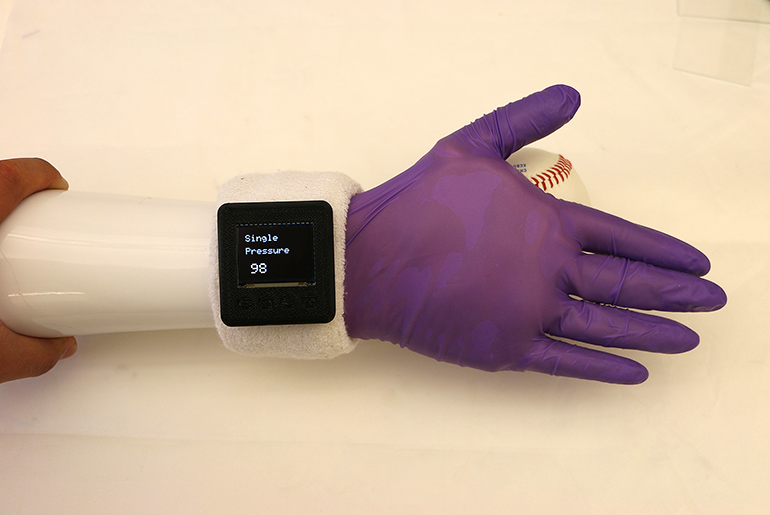
Read MoreMulti-Sensing Glove Makes Prosthetic Hands More Real
Engineers from Purdue University, University of Georgia, and University of Texas have combined forces to develop a glove that can be put over existing prosthetic hands to give them a more life-like feel and the ability to sense a variety of parameters. The glove is intended to improve a user’s ability to interact with others.…
Read MoreFirst Long-distance Heart Surgery Performed via Robot
A doctor in India has performed a series of five percutaneous coronary intervention (PCI) procedures on patients who were 20 miles away from him. The feat was pulled off using a precision vascular robot developed by Corindus. The results of the surgeries, which were successful, have just been published in EClinicalMedicine, a spin-off of medical…
Read More