Archive for February 2020
Cerus Endovascular Receives CE Mark Approval for its Contour Neurovascular System, Designed to Treat Intracranial Aneurysms
Cerus Endovascular Ltd., a privately-held, commercial stage medical device company, today announced that it has received CE Mark approval for its lead product, the Contour Neurovascular System™, for the treatment of intracranial aneurysms. The Contour Neurovascular System™ is a unique, fine mesh braid that is deployed across the neck of the aneurysm sac and provides…
Read MoreCrossRoads Extremity Systems, LLC Announces Launch of the HiMax Plus Widebody Fixation System for Foot Fusions
CrossRoads® Extremity Systems, the global leader in Staple Compression Plates(SCP) and nitinol technology for the lower extremities, announced it has received FDA clearance and launched the HiMax® Plus Widebody Fixation System. HiMax® Plus is the only foot bone fusion system on the market to provide flush insertion, market leading compression, and a wide bridge for enhanced stability. The features work…
Read MoreBaxter and COSMED Announce U.S. FDA 510(k) Clearance of Q-NRG+ Indirect Calorimetry Device
Baxter International Inc. (NYSE: BAX), a global leader in clinical nutrition, today announced the U.S. Food and Drug Administration (FDA) clearance of Q-NRG+, a metabolic monitoring device utilizing indirect calorimetry (IC) technology. IC is considered the “gold standard”1 to accurately measure a patient’s calorie needs, or resting energy expenditure (REE). These readings can help inform prescription…
Read MoreCarelight Introduces Line Of FDA-Cleared Light Therapy Devices
CareLight Solutions is proud to now be offering a complete line of industry-leading Light Therapy products to the public. Inspired by Nobel Prize-winning science, Light Therapy devices are backed by years of scientific research and are FDA-cleared to reduce pain and increase circulation. Based in Chicago, CareLight is dedicated to the health and well-being of its…
Read MoreMEDICREA Announces FDA Clearance of the World’s First Patient-Matched Spinal Interbody Cages
MEDICREA® (Euronext Growth Paris : FR0004178572 – ALMED, PEA-PME eligible and OTCQX : MRNTF), pioneering the transformation of spinal surgery through Artificial Intelligence, predictive modeling and patient specific implants with its UNiD® ASI (Adaptive Spine Intelligence) proprietary software platform, concierge expert services and technologies, announced today that it has received FDA-Clearance for UNiD® IB3D Patient-Matched interbody cages which…
Read MoreWorld’s First Bedside MRI System Receives FDA 510(k) Clearance
Hyperfine Research, Inc. announced today that it has received US Food & Drug Administration 510(k) clearance for the world’s first bedside Magnetic Resonance Imaging (MRI) system, clearing the way for device shipments this summer. The Hyperfine system is 20X lower cost, 35X lower power consumption, and 10X lower weight than today’s fixed conventional MRI systems.…
Read MoreIntuitive Acquires Orpheus Medical to Expand Informatics Platform for Hospitals
Intuitive, a global technology leader in minimally invasive care and the pioneer of robotic-assisted surgery, announced it has acquired privately held Orpheus Medical to deepen and expand its integrated informatics platform. Orpheus Medical provides hospitals with information technology connectivity, as well as expertise in processing and archiving surgical video. “The addition of Orpheus will provide…
Read MoreFDA Grants De Novo Clearance to Bluegrass Vascular Technologies for the Surfacer Inside-Out Access Catheter System
Bluegrass Vascular Technologies (Bluegrass Vascular), a private medical technology company focused on innovating lifesaving devices and methods for vascular access procedures, announced today that the U.S. Food and Drug Administration (FDA) has granted a De Novo classification order for its Surfacer® Inside-Out® Access Catheter System. The Surfacer System is intended to obtain central venous access to facilitate catheter insertion into…
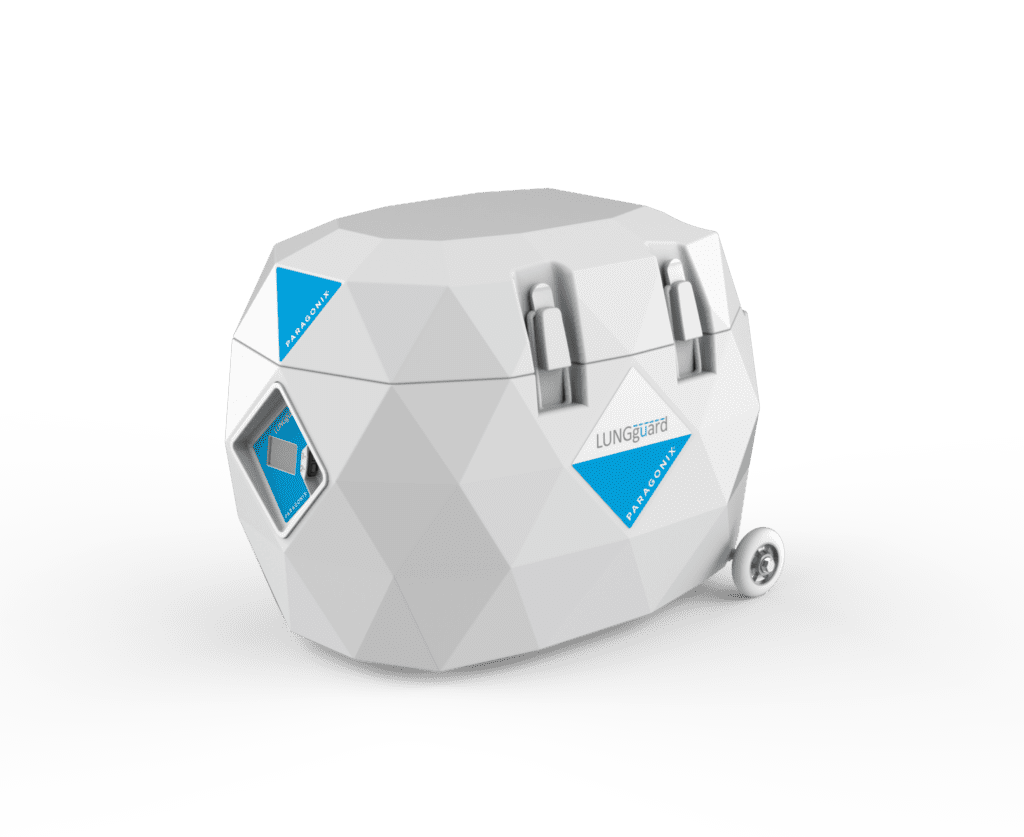
Read MoreFDA Clears Paragonix Technologies’ LUNGguard for Donor Lung Preservation
Paragonix Technologies, Inc. today announced clearance of a Pre-Marketing Notification (510(k)) with the US Food and Drug Administration (FDA) for its Donor Lung Preservation System – LUNGguard1,2. The Paragonix LUNGguard Donor Lung Preservation System is intended to be used for the static hypothermic preservation of lungs during transportation and eventual transplantation into a recipient using cold…
Read MoreIn2Bones Receives FDA 510(k) Clearance for Quantum Total Ankle
In2Bones Global, Inc. today announces clearance from the U.S. Food and Drug Administration to market the Quantum™ Total Ankle. This new total ankle replacement system treats patients who suffer from arthritis and is designed to improve patient mobility, increase stability, and technologically advance implant placement based on patient-specific anatomy. Compared to current total ankles on the…
Read More