Archive for May 2020
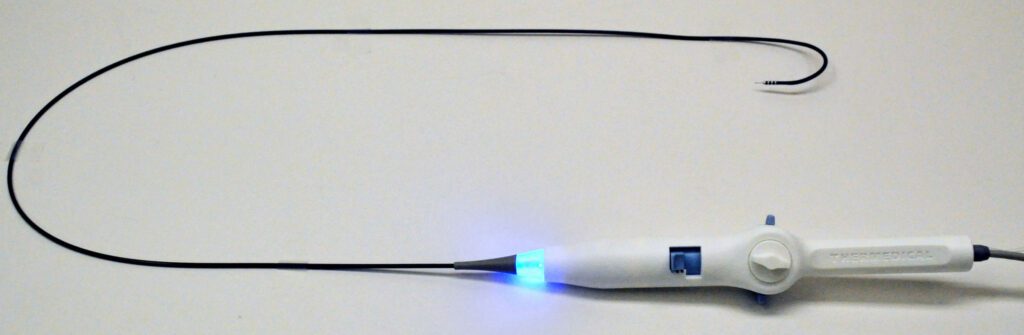
Thermedical’s Groundbreaking SERF Ablation System Earns FDA’s Breakthrough Device Designation
Thermedical®, a developer of thermal-ablation medical systems to treat ventricular tachycardia (VT), today announced that it has received Breakthrough Device Designation from the U.S. Food & Drug Administration (FDA) for its Saline Enhanced Radiofrequency (SERF) Ablation system and Durablate® catheter. The FDA Breakthrough Devices Program is intended to help patients receive more timely access to technologies that…
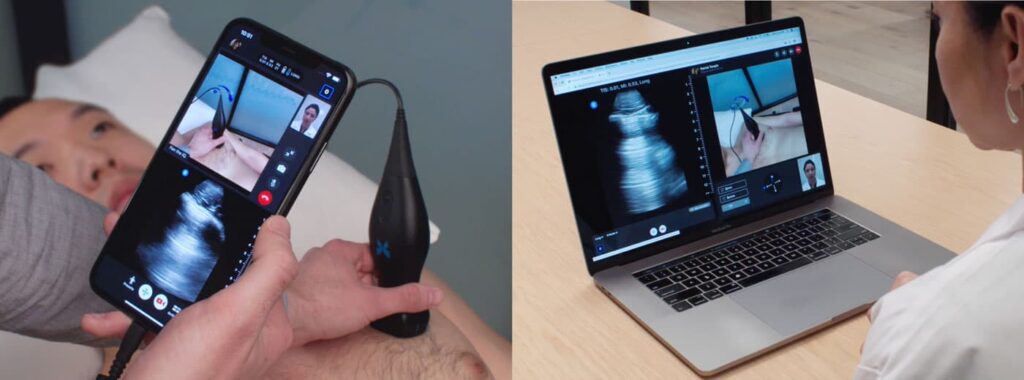
Read MoreButterfly Network, Inc. is Bringing Ultrasound into the Home with Telemedicine
Butterfly Network has developed and is rolling out a new version of TeleGuidance™, their ultrasound-based telemedicine platform. TeleGuidance brings medical imaging to telemedicine. It leverages an array of leading-edge and easy-to-use augmented reality guiding tools. It allows trained practitioners to perform an ultrasound scan without being next to the patient. “We are grateful for the…
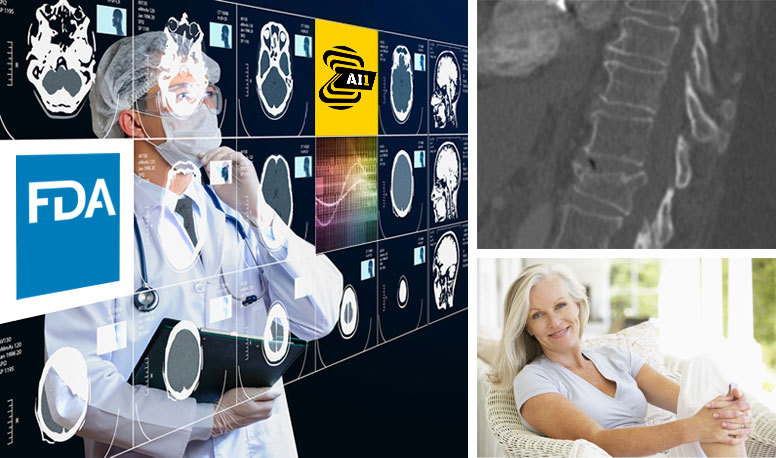
Read MoreZebra Medical Vision Secures its 5th FDA Clearance, Making Its Vertebral Compression Fractures AI Solution Available in the U.S.
Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its fifth FDA 510(k) clearance for its Vertebral Compression Fractures (VCF) product. The company’s latest AI solution automatically identifies findings suggestive of compression fractures, enabling clinicians to place patients that are at risk of osteoporosis in treatment pathways to prevent potentially life-changing fractures. The…
Read MoreFDA and CDC Drawing Up Plan to Restart Routine Facility Inspections
Amid the coronavirus outbreak reaching pandemic level in March, FDA stopped routine inspections of domestic and international facilities. Under the revised policy, the agency would perform inspections seen as “mission critical” while relying on off-site monitoring and the commitment of companies to quality in the absence of in-person regulatory oversight to maintain standards during the…
Read MoreNevro Announces CE Mark Approval of Senza Omnia Spinal Cord Stimulation System to Treat Chronic Pain
Nevro Corp., a global medical device company that is providing innovative, evidence-based solutions for the treatment of chronic pain, today announced it has received CE mark approval for the Senza® Omnia™ Spinal Cord Stimulation (SCS) system. “Following a successful launch in the United States late last year, we are excited to now have approval for the Senza Omnia…
Read MoreAbbott Receives FDA Emergency Use Authorization for COVID-19 Antibody Blood Test on Alinity i System
Abbott announced today that the U.S. Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for the company’s SARS-CoV-2 IgG lab-based serology blood test on the Alinity™ i system. Abbott plans to ship nearly 30 million antibody tests globally in May across its ARCHITECT® and Alinity i™ platforms and will have capacity for 60 million tests in…
Read MoreFDA Issues Emergency Use Authorization for Eko’s ECG-based Low Ejection Fraction Screening Algorithm, Designed to Improve Detection of Heart Failure During COVID-19 Pandemic
Eko, a digital health company building AI-powered screening and telehealth solutions to fight cardiovascular disease, today announced that the U.S. Food and Drug Administration (FDA) has issued the company an Emergency Use Authorization (EUA) for its novel ECG-based algorithm that can provide an easily accessible, rapid screening test for low ejection fraction (low EF), a weak heart…
Read MoreAmbu Launches New Single-Use Cystoscope for Improved Workflow and Enhanced Productivity Benefits
Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, introduced today a new single-use cystoscope. The Ambu® aScope™ 4 Cysto will give urologists immediate access to a single-use cystoscope that can be used for procedures such as bladder cancer surveillance, stent removal and other common cystoscopy procedures. Built on more…
Read MoreCardioFocus Announces US FDA Approval of HeartLight X3 System for the Treatment of Atrial Fibrillation
CardioFocus, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved the next-generation HeartLight® X3 Endoscopic Ablation System for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF). Approval of the HeartLight X3 System came as a result of a comprehensive submission, including outcomes from the study of 60 HeartLight X3 patients. In…
Read MoreCleanbox Technology to Help Combat COVID-19 PPE Shortage by Allowing Safe Reuse of N95 Masks
Smart tech hygiene company Cleanbox Technology, Inc. has announced a new product, the CleanDefense™, a point-of-use mask decontamination product in response to SARS CoV-2 (COVID-19). Cleanbox’s patented technology uses UVC light in an LED to decontaminate hard-to-clean products worn on the face or head. With a focus on high risk contagion transfer points, its core products were designed…
Read More