Archive for May 2020
FDA Approves Aspenstate’s AiRTouch Portable X-Ray System for Human Medical Use
Aspenstate has received clearance for their portable x-ray system, AiRTouch, from the U.S. Food and Drug Administration (FDA). Amongst many applications, this device is a simple and efficient front-line tool to acquire chest x-rays to diagnose COVID-19. The AiRTouch device offers a unique list of capabilities in the battle against COVID-19. It’s lightweight (5.5 lbs),…
Read More3M Awarded Department of Defense Contracts to Further Expand U.S. Production of N95 Respirators
3M has been awarded two contracts through the U.S. Department of Defense in recent weeks to further expand U.S. production of N95 respirators in response to the COVID-19 outbreak. Beginning in January, 3M ramped up production of respirators and doubled its global output to 1.1 billion per year – including 35 million N95 respirators per…
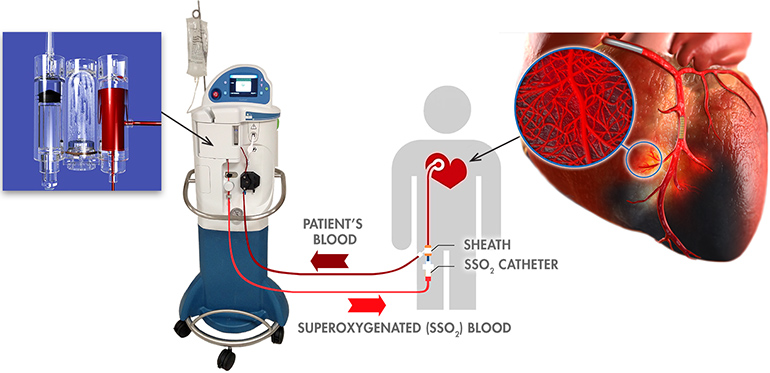
Read MoreZOLL TherOx Receives CE Mark Approval for Supersaturated Oxygen Therapy
ZOLL Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today it has received CE Mark approval to market and distribute its SuperSaturated Oxygen (SSO2) Therapy System in Europe. SSO2 Therapy provides interventional cardiologists with the first and only clinically proven treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage…
Read MorePulmonx Secures $66M in Financing to Support Commercial Acceleration of the Zephyr Valve System, a Minimally-Invasive Treatment for Severe Emphysema
Pulmonx Corporation, a commercial-stage medical technology company that provides a minimally-invasive treatment for patients with severe emphysema, announces a $66 million financing led by Ally Bridge Group, a leading global life science investor. The financing also attracted new investors Adage Capital Management, HealthQuest Capital, Partner Fund Management, and Rock Springs Capital, as well as existing…
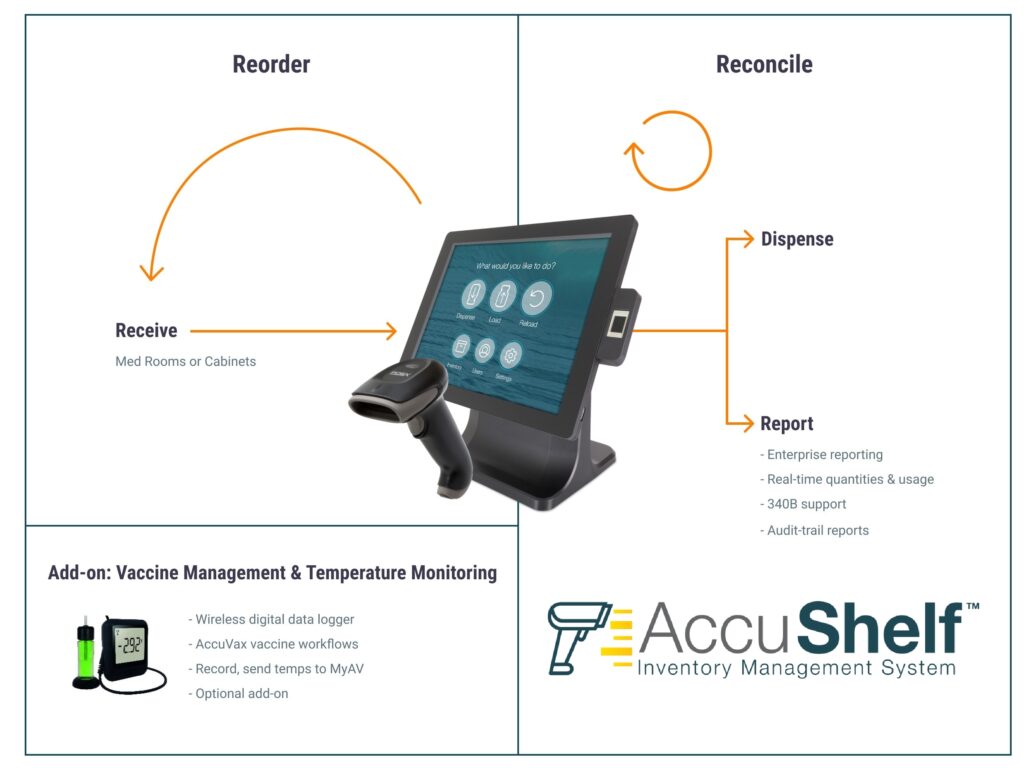
Read MoreTruMed Systems, Inc. Announces Launch of the AccuShelf Total Practice Inventory Management System, to Complement the AccuVax Vaccine Storage and Handling Solution
AccuShelf makes inventory control simple, from tracking of receiving items, to patient administration. The system utilizes an all-in-one touch screen PC system with an integrated biometric reader and a wireless barcode scanner to capture and record every medication dose with lot, and expiration. It tracks each dose to a specific patient and can communicate with…
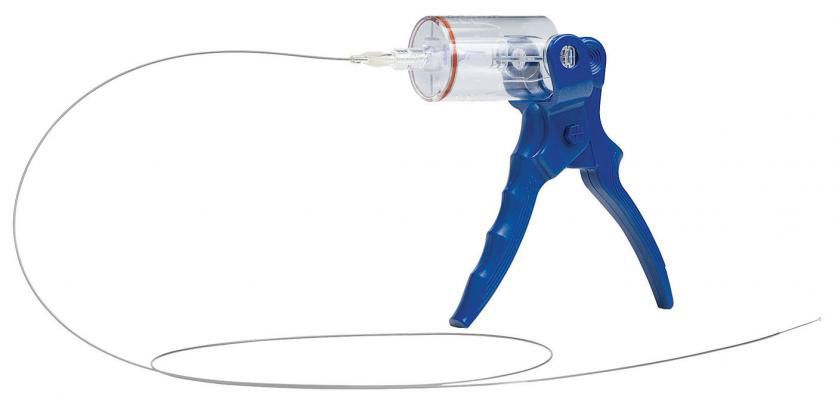
Read MoreFDA Clears New Mechanical Thrombectomy Platform
Control Medical Technology announced the FDA cleared the Aspire MAX 7 – 11F Mechanical Thrombectomy platform to remove blood clots from peripheral vessels. Blood clot removal (thrombectomy) is a common procedure. Coronary thrombectomy is associated with acute myocardial infarction (AMI), neurovascular thrombectomy is associated with acute ischemic stroke, and peripheral thrombectomy is associated with peripheral…
Read MoreFDA Grants Emergency Use Authorization to VitalConnect for Cardiac Monitoring in COVID-19 Patients
VitalConnect®, Inc., a leader in wearable biosensor technology, announced it was granted Emergency Use Authorization (EUA) status by the U.S. Food and Drug Administration (FDA) as part of the response to the COVID-19 pandemic. The FDA EUA will further enhance the capabilities of the VitalPatch and continuous patient monitoring technology, the Vista Solution. Under the FDA EUA, the…
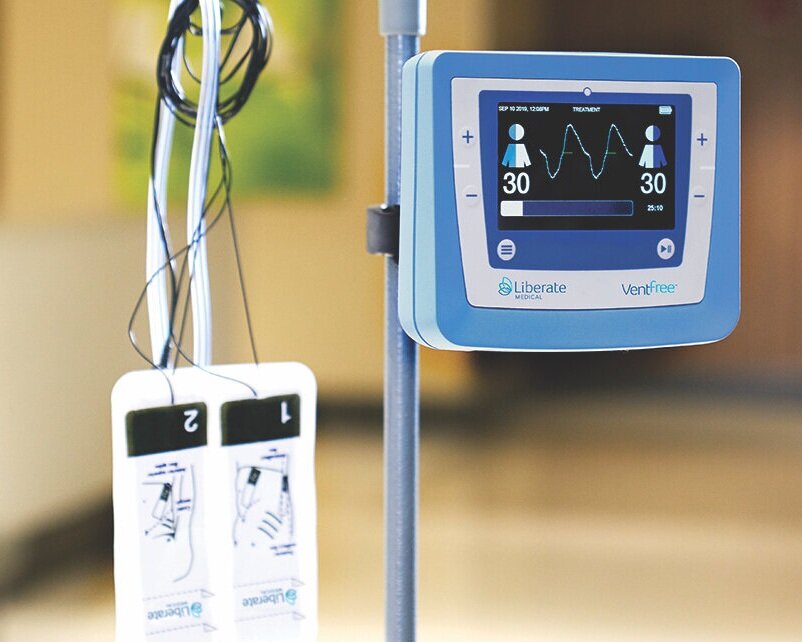
Read MoreVentFree Respiratory Muscle Stimulator Receives FDA Emergency Use Authorization for Use During COVID-19 Pandemic
Liberate Medical today announced that it has received Federal Drug Administration (FDA) Emergency Use Authorization for its VentFree™ Respiratory Muscle Stimulator, intended to be used to reduce disuse atrophy of the abdominal wall muscles, which may reduce the number of days adult patients require mechanical ventilation, including those patients with COVID-19. Reducing the time patients…
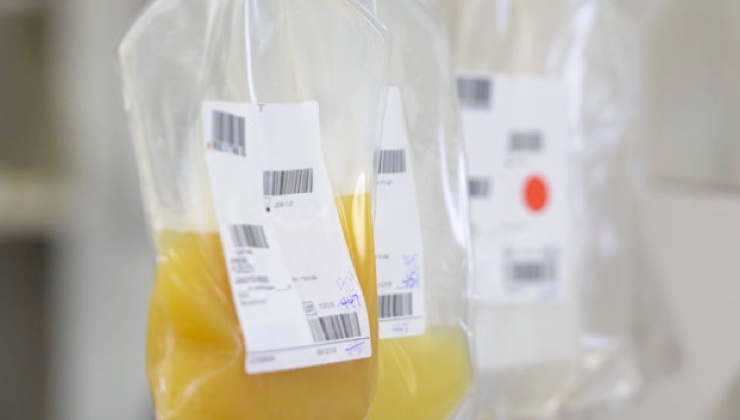
Read MoreCerus Corporation Announces FDA Approval for INTERCEPT Blood System for Plasma with Alternate Plastic Disposable Kits
Cerus Corporation (Nasdaq:CERS) announced today FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative plastic disposable kit. The planned conversion to these new kits is part of the Company’s ongoing strategy to enhance its global supply chain integrity that was initiated several years ago. “The FDA approval for the new INTERCEPT plasma…
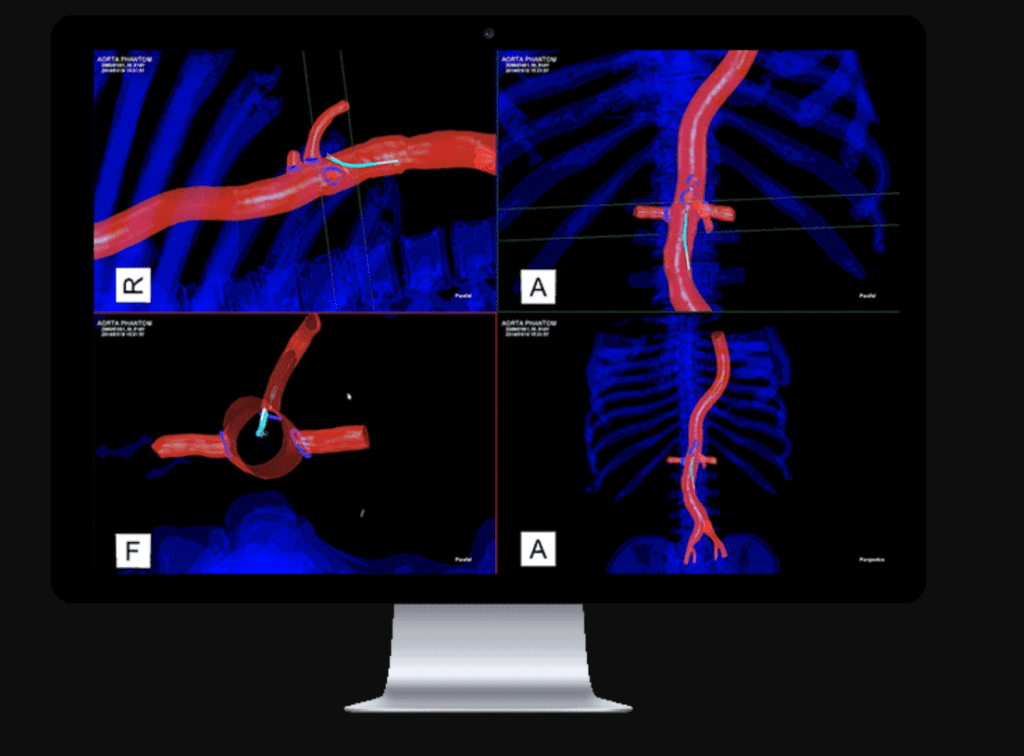
Read MoreCenterline Biomedical Completes First Human Patient in the United States with IOPS (Intra-Operative Positioning System)
Surgical navigation startup Centerline Biomedical, Inc. (Centerline), a Cleveland Clinic spin-off company, has announced the successful completion of the first in a series clinical cases in the United States as it begins limited launch of its innovative IOPS™ technology platform. The FDA-cleared technology is being deployed at Cleveland Clinic to begin, with other leading intuitions to follow,…
Read More