Archive for June 2023
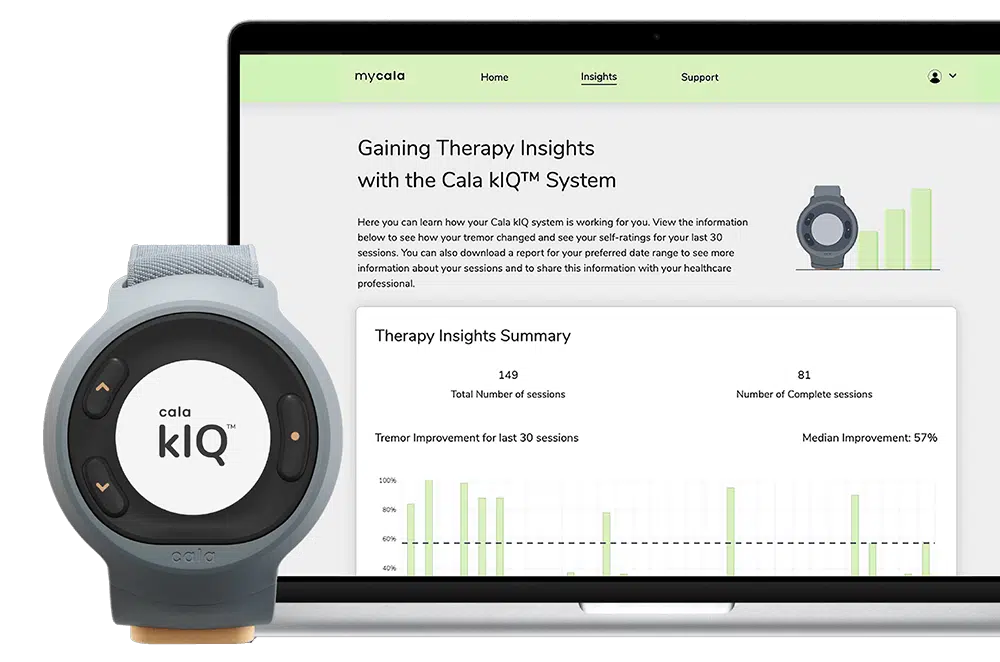
Cala® Launches The Cala kIQ™ System, Offering Meaningful Tremor Relief for Patients With Essential Tremor and Now Parkinson’s Disease
Cala, the bioelectronic medicine leader setting a new standard of care for chronic disease, today announced the commercial launch of its next generation system: the Cala kIQ™ System, the first and only FDA-cleared wearable device that delivers effective therapy for action hand tremor relief in people with essential tremor and Parkinson’s disease. The Cala kIQ…
Read MoreSurmodics Receives FDA 510(k) Clearance for Pounce™ LP Thrombectomy System
Surmodics, Inc. (Nasdaq: SRDX), a leading provider of medical device and in vitro diagnostic technologies, announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Pounce™ LP (Low Profile) Thrombectomy System. Introduced in 2021, the Pounce Thrombectomy System is intended for the non-surgical removal of thrombi and emboli from the peripheral…
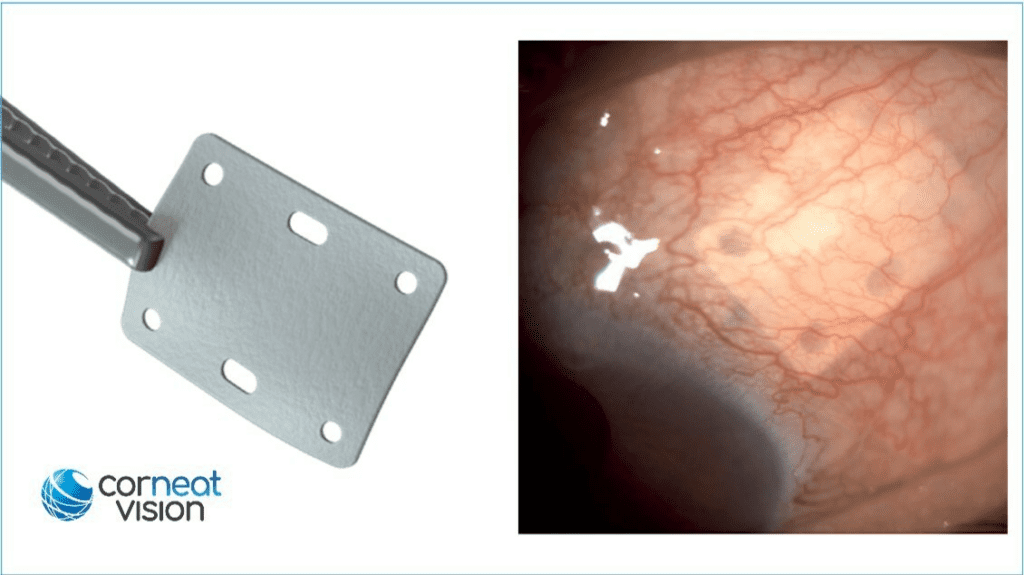
Read MoreFDA Clears CorNeat EverPatch, World’s First Non-degradable, Synthetic Tissue Substitute for Ophthalmic Surgery
CorNeat Vision’s EverPatch, a synthetic tissue substitute, has been granted 510(k) clearance by the US Food & Drug Administration (FDA). The CorNeat EverPatch is the first synthetic, non-degradable tissue-integrating matrix for use in ophthalmic surgeries. It is composed of a non-woven, polymer matrix which integrates with surrounding tissue and is intended to reinforce the sclera and…
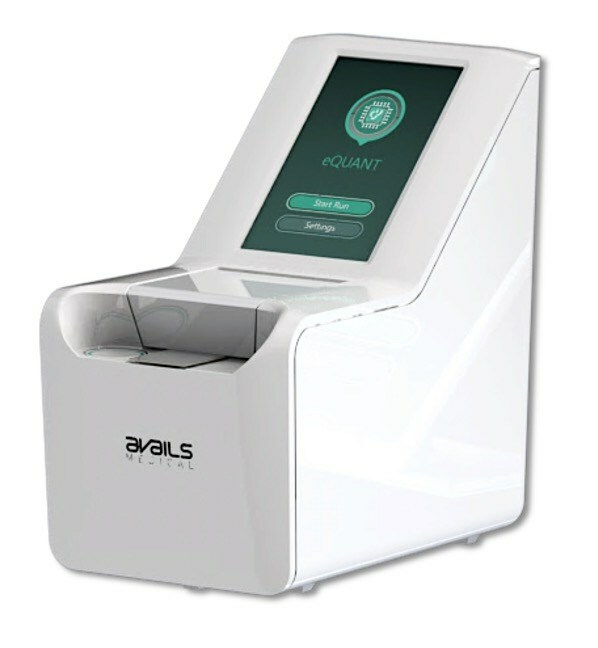
Read MoreAvails Medical’s eQUANT™ System Submitted to FDA for 510(k) Clearance
Avails Medical, a pioneer in rapid, automated and fully electrical antibiotic susceptibility testing (AST) solutions announced today the submission of its eQUANTTM system for FDA 510(k) clearance. The eQUANT system provides a standardized inoculum (0.5 McFarland equivalent) directly from a positive blood culture, which is designed to be used with traditional automated AST systems and Disk…
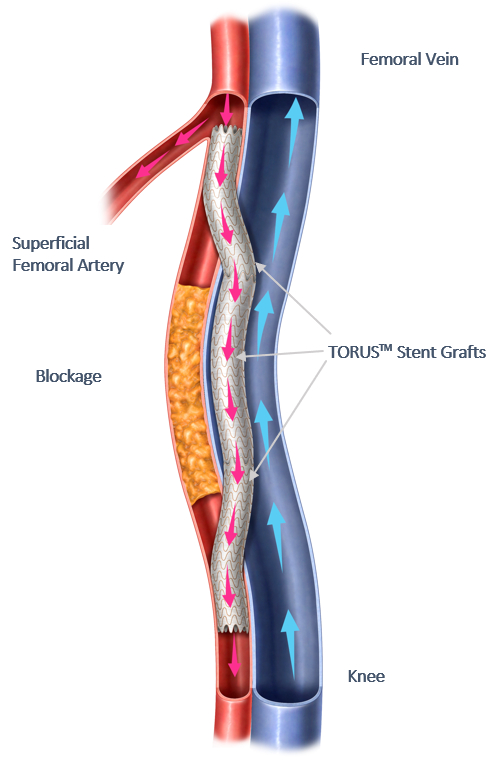
Read MoreEndologix Receives FDA Approval of the DETOUR™ System to Treat Long Complex Superficial Femoropopliteal Lesions in Patients with PAD
Endologix LLC, a privately held, global medical device company dedicated to providing disruptive therapies for the interventional treatment of vascular disease, announced today that the U.S. Food and Drug Administration (FDA) has granted approval for the DETOUR System to treat patients with complex peripheral arterial disease (PAD). Over 8.5 million Americans are affected by PAD.…
Read MoreInnova Vascular Earns FDA Clearance for Two New Thrombectomy Devices: Laguna Clot Retriever™ System and Malibu Aspiration Catheter™ System
Innova Vascular, Inc. announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Laguna Clot Retriever™ System and its Malibu Aspiration Catheter™ System for use in the peripheral vasculature. The two new devices are collectively known as the Laguna Thrombectomy System. “Removing clots quickly, safely, and in large volumes from the…
Read MoreArtelon, Inc.’s FlexBand®, FlexPatch®, and FlexBand Plus® earn FDA Clearance for Ligament Reinforcement
Artelon Inc., announced today U.S. FDA 510(k) clearance of FlexBand®, FlexPatch®, and FlexBand Plus® for ligament repair surgery in addition to tendon repairs*. This new clearance expands the indications for these products to now include reinforcement of medial, lateral and ulnar collateral ligaments, spring ligaments, deltoid ligaments, and extra-articular ligaments in the ankle, knee, and…
Read MoreWaters Takes Targeted, Quantitative Imaging to the Next Level with New DESI Source for the Xevo TQ Absolute System
American Society for Mass Spectrometry (ASMS) — Waters Corporation (NYSE:WAT) today launched the industry’s first targeted imaging mass spectrometer based on its Xevo™ TQ Absolute tandem quadrupole mass spectrometer, the most sensitive and compact mass spectrometer in its class. The new instrument combines the Waters™ DESI XS source with the Xevo TQ Absolute system and is five times more sensitive and five…
Read More