Archive for March 2020
Intuitive Surgical Among 1st Big Medtech Companies to Warn of Costly Pandemic Disruptions
Intuitive said the disruptions it’s felt to date in China, Korea, Taiwan and Italy “do not represent a material portion of our current procedure volume.” n Intuitive’s most critical U.S. market, infections are rising and rapid implementation of mitigation strategies more tangibly threatens the company’s goals for the year. During its most recent earnings call…
Read MoreTransEnterix Receives FDA Clearance for First Machine Vision System in Robotic Surgery
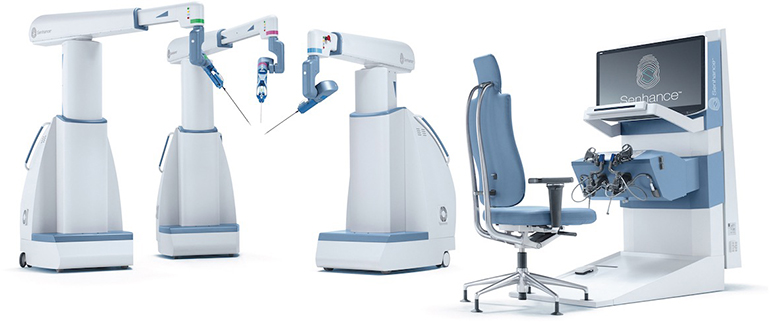
TransEnterix, Inc., a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced the Company received 510(k) clearance for the Intelligent Surgical Unit (ISUTM) that enables machine vision capabilities on the SenhanceⓇ Surgical System. “We are pleased to have received this important clearance earlier than…
Read MoreFDA Clearance Brings RefleXion Closer to Expanding Cancer Treatment Market
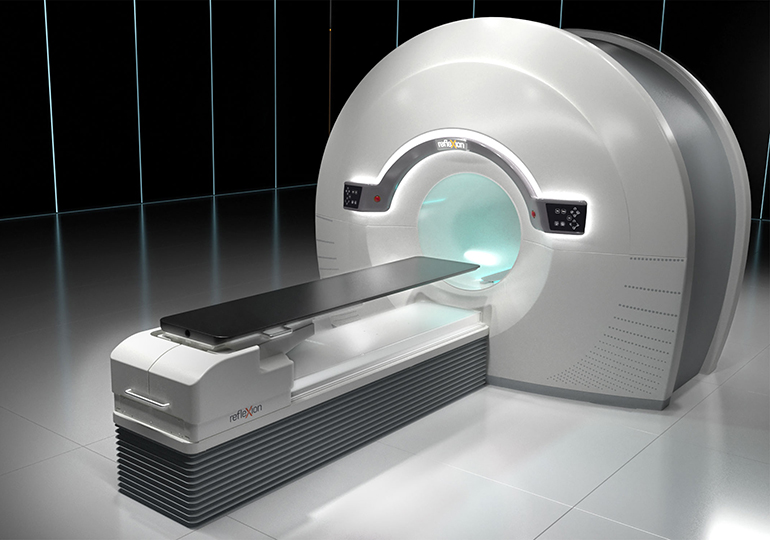
RefleXion Medical, a therapeutic oncology company pioneering the use of biology-guided radiotherapy (BgRT)* to treat all stages of cancer today announced it has received marketing clearance from the U.S. Food & Drug Administration (FDA) for stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT) for its RefleXion™ X1 machine. “We are at the…
Read MoreHologic’s Molecular Test for the Novel Coronavirus Receives FDA Emergency Use Authorization
Hologic, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for the Company’s new Panther Fusion® SARS-CoV-2 assay, a molecular diagnostic test that detects SARS-CoV-2, the virus that causes COVID-19 disease. Hospital, public health and reference laboratories can perform the test on Hologic’s Panther Fusion system, a fully automated,…
Read MoreThe Consult Station: Front-Line Detection and Triage to Contain the Spread of Covid-19
H4D, a leading global telemedicine company, is joining the international effort to fight the global coronavirus pandemic. H4D is providing hospitals with a fast, efficient and reliable solution to improve patient flow management in the emergency department. The Consult Station® allows the 4 key vital parameters to be taken in 5 minutes, rapidly identifying the…
Read MoreEndologix Announces FDA Approval of AFX Endovascular AAA System
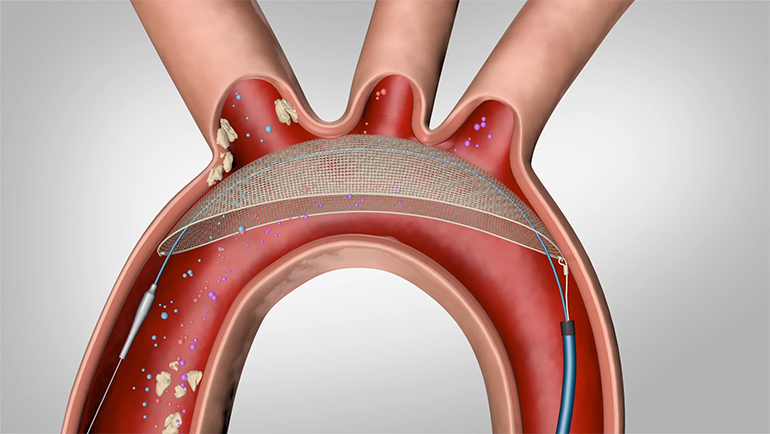
Endologix, Inc. (Nasdaq: ELGX), developer and marketer of minimally invasive treatments for aortic disorders, announced today that it has received U.S. Food and Drug Administration (FDA) approval for its next generation product, the AFX™ Endovascular AAA System, for the treatment of abdominal aortic aneurysms (AAA). Endologix is introducing AFX at the Annual Meeting of the Society…
Read MoreTriGUARD 3 Cerebral Embolic Protection Device Receives CE Mark for Use in Transcatheter Heart Procedures
Keystone Heart Ltd., a Venus Medtech Company, today announced European CE Mark for the TriGUARD 3™ Cerebral Embolic Protection (CEP) Device. The device is designed to minimize the risk of cerebral damage by deflecting embolic debris away from cerebral circulation during Transcatheter Aortic Valve Implantation (TAVI) and other transcatheter heart procedures. “Even with increased operator experience…
Read MoreFDA Clears Fluidda’s Broncholab Platform for Use in Clinical Practice
The US Food and Drug Administration provided market clearance to Fluidda for its Broncholab platform. Broncholab provides a number of Functional Respiratory Imaging (FRI) parameters to physicians via an online platform to assist in diagnosing and monitoring of respiratory diseases. The severity of respiratory diseases is often underestimated by conventional lung function tests, such as…
Read MoreOSSIO Receives FDA 510(k) Clearance for OSSIOfiber Hammertoe Fixation System
OSSIO, Inc., an orthopedic fixation company, today announced that its OSSIOfiber® Hammertoe Fixation System has received 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for maintenance of alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts. These novel bio-integrative implants with disposable, sterile instrumentation now come in three sizes, with…
Read MoreCryoLife Receives CE Mark for E-vita OPEN NEO
CryoLife, Inc., a leading cardiac and vascular surgery company focused on aortic disease, announced today that it has received CE Mark for the E-vita Open NEO, a hybrid stent graft system for the treatment of aortic arch disease. Aortic arch disease includes both aortic aneurysms and aortic dissections, which occur suddenly and usually without warning. Approximately…
Read More