Archive for March 2020
NeuroPace RNS System Receives FDA Approval for MRI Labeling, Allowing Thousands More Patients to Benefit from Personalized, Data-Driven Epilepsy Treatment
NeuroPace, Inc., a Silicon Valley-based medical technology company, today announced that its RNS® System has received U.S. Food and Drug Administration (FDA) approval of MRI labeling for the RNS System, expanding treatment options for the approximately one million patients in the United States living with seizures that do not respond to medication. Individuals with focal onset…
Read MoreHologic Receives CE Mark for Three-in-One Omni Hysteroscope in Europe
Hologic, Inc. (Nasdaq: HOLX) announced today that it has received a CE mark in Europe for its Omni™ hysteroscope, an innovative three-in-one modular scope with advanced visualization capabilities designed for both diagnostic and therapeutic hysteroscopic procedures. Obstetricians and gynecologists (ObGyns) can use the new Omni hysteroscope in out- and in-patient settings. “Experts agree that direct visualization…
Read MoreTriMed, Inc. Announces Release of the ASET™ Foot Plating Systems
TriMed, Inc. an orthopaedic medical device developer and manufacturer focused on extremities, announced it would begin marketing its new ASET™ Foot Plating System. The ASET Foot Plating System, designed to treat common forefoot and mid-foot indications, offers unique implants to treat MTP fusions, Lapidus fusions, and TMT fusions. In addition, the system’s variety of patented…
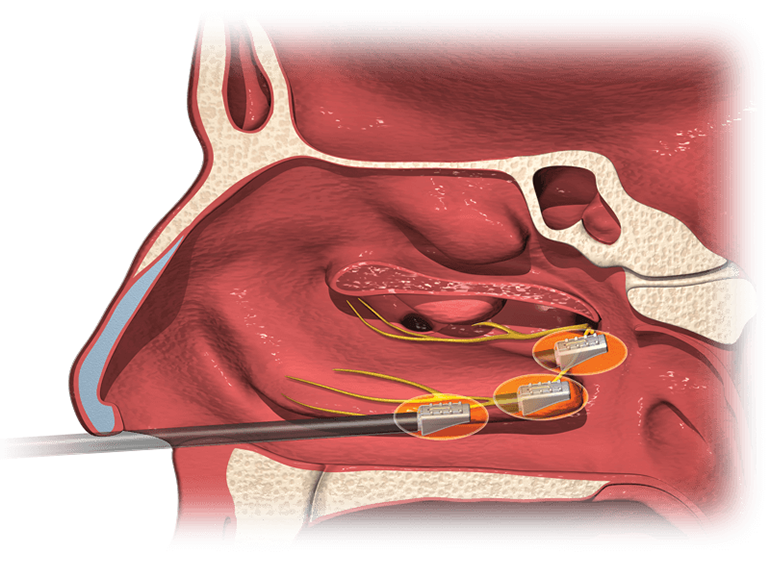
Read MoreAerin Medical Announces FDA Clearance and U.S. Launch of Innovative Nonsurgical Procedure for Chronic Rhinitis
Aerin Medical Inc., a company focused on minimally invasive solutions for chronic nasal conditions, today announced U.S. Food and Drug Administration (FDA) clearance and U.S. launch of the company’s second product, the RhinAer™ Stylus, an innovative device for nonsurgical treatment of chronic rhinitis. More than 30 million Americans suffer from nonallergic rhinitis.1 Patients with the condition…
Read MoreHIMSS Announces Cancellation of the 2020 Global Health Conference & Exhibition
Today, following recent reports from the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), HIMSS announced it is clearly necessary to cancel the 2020 HIMSS Global Health Conference & Exhibition. “We recognize all the hard work that so many have put into preparing for their presentations and panels that accompany every HIMSS conference,” said Hal Wolf,…
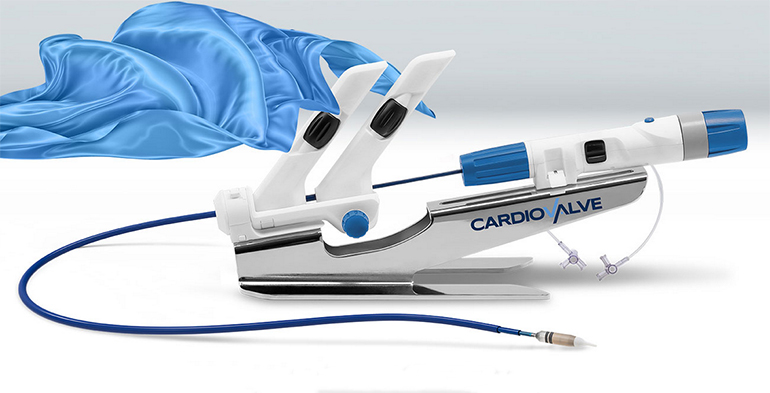
Read MoreCardiovalve Wins FDA Breakthrough Device Designation for Transcatheter Tricuspid Valve Replacement System
Cardiovalve announced that it has received U.S. Food and Drug Administration (FDA) approval for an Early Feasibility Study (EFS) of its Transcatheter Tricuspid Valve Replacement System for a tricuspid regurgitation (TR) indication. The Cardiovalve System also has been granted ‘Breakthrough Device Designation’ status by the FDA. Cardiovalve is the first privately held company to have…
Read MoreFDA Bans Certain Electrical Stimulation Devices, the Third Ban in History
FDA has officially banned electrical stimulation devices used to treat self-injury or aggressive behavior with publication of a final rule Wednesday. The action comes almost six years after convening an advisory committee to weigh the ban and four years after actually proposing it, as pressure mounted from lawmakers in recent months to follow through on…
Read MoreAltair Medical Awarded FDA Breakthrough Device Status to Address the Global Opioid Crisis
Altair Medical, a medical technology company developing a groundbreaking solution to the global opioid crisis, today announce that the US Food and Drug Administration (FDA) has awarded the Company Breakthrough Device designation for its RESPMETERTM wearable biosensor device. Altair Medical’s RESPMETERTM is a chest-worn wireless sensor that accurately detects when someone is suffering Opioid Indused Respiratory Depression…
Read MoreXaTek’s ClotChip Earns FDA’s Breakthrough Device Designation
XaTek Inc. today announced that the company has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its in-development ClotChip, marking a significant and distinguishing step in advancing the company’s life-changing portable blood-clotting sensor toward commercialization. The FDA Breakthrough Devices Program was created in 2018 to expedite the development, assessment and…
Read MoreNanox Signs Agreement With The Gateway Group for the Deployment of 1,000 Nanox.ARC Units Across Australia, New Zealand, and Norway
NANO-X IMAGING LTD (www.nanox.vision) (“Nanox” or the “Company”), an innovative medical imaging technology company, announces it has secured an exclusive distribution deal with The Gateway Group (“Gateway”), one of Australia’s largest independent product distributors including health, wellness, medical supplies and devices. The agreement has an initial term of three years and is renewable for an additional term of…
Read More