Archive for August 2023
Welldoc Receives 10th 510(k) Clearance from FDA for Award-Winning Diabetes Platform BlueStar®
Welldoc®, a digital health leader revolutionizing chronic care, today announced the receipt of its 10th 510(k) clearance from the Food and Drug Administration (FDA) for its award-winning diabetes digital health solution, BlueStar®. This clearance reinforces Welldoc’s position as a leader in technology in diabetes management. This 10th 510(k) clearance enables BlueStar to use connected insulin dosing…
Read MoreCeribell Receives FDA 510(k) Clearance and CMS NTAP Reimbursement for New ClarityPro™ Software with Electrographic Status Epilepticus Diagnostic Indication
Ceribell, Inc.® announced that its new software ClarityPro has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the indication of diagnosing Electrographic Status Epilepticus (ESE). The clearance follows prior receipt of Breakthrough Device Designation from FDA. Subsequent to receiving Breakthrough Device Designation and 510(k) clearance from FDA, the U.S. Centers for Medicare…
Read MoreViseon Inc. Announces Commercial Rollout and Initial Clinical Use of the 4K MaxView® System for Advanced Digital Visualization During Minimally Invasive Spine Surgery
Viseon Inc. announced the US commercial rollout and initial clinical use of the First-of-its-Kind 4K Advanced Visualization System for Minimally Invasive Spine Surgery. The Viseon MaxView 4K System is an enabling, towerless, state-of-the-art, advanced visualization system involving no capital equipment expense and occupying no operating room footprint, most accommodating in an ASC setting. The 4K…
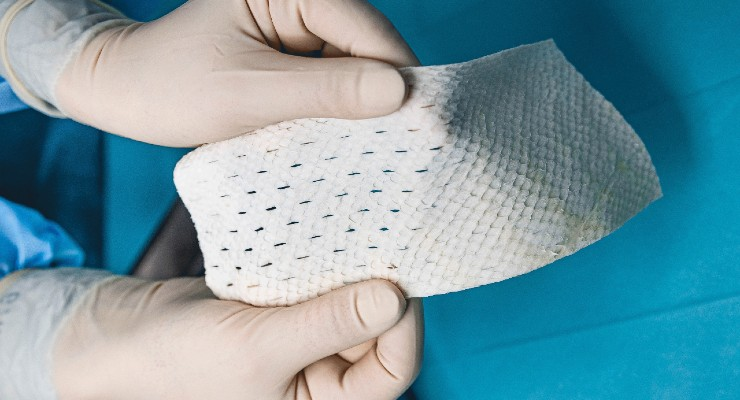
Read MoreKerecis to Donate FDA-Approved Fish Skin for Burn Victims of Maui Wildfires
Kerecis®, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, is donating its GraftGuide® fish-skin burn product for victims of the fires on Maui, Hawaii. Qualified medical personnel wanting to get fish-skin burn-treatment products for their patients should call 703-287-8752 or email wildfires@kerecis.com. Kerecis is dispatching a specialist to train…
Read MoreAcorai receives Breakthrough Device Designation for their Non-Invasive Intracardiac Pressure Monitor
Acorai, a start-up medical device manufacturer from Sweden, today announces that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for Acorai’s Heart Monitor, a device for the non-invasive estimation of diastolic pulmonary artery pressure (dPAP), systolic pulmonary artery pressure (sPAP), and mean pulmonary artery pressure (mPAP) in patients with Stage C Heart…
Read MoreCapstan Medical Leverages Robotics to Bring Minimally Invasive Care to Heart Valve Patients
Capstan Medical, a developer of minimally invasive technology to address heart valve disease, today announced an oversubscribed $31.4 million Series B investment led by Eclipse. Additional participating investors include Intuitive Ventures and Puma Venture Capital, a new firm founded by Amit Hazan, a veteran Wall Street medical technology analyst, and Dr. Vipul Patel, a renowned robotic surgeon who…
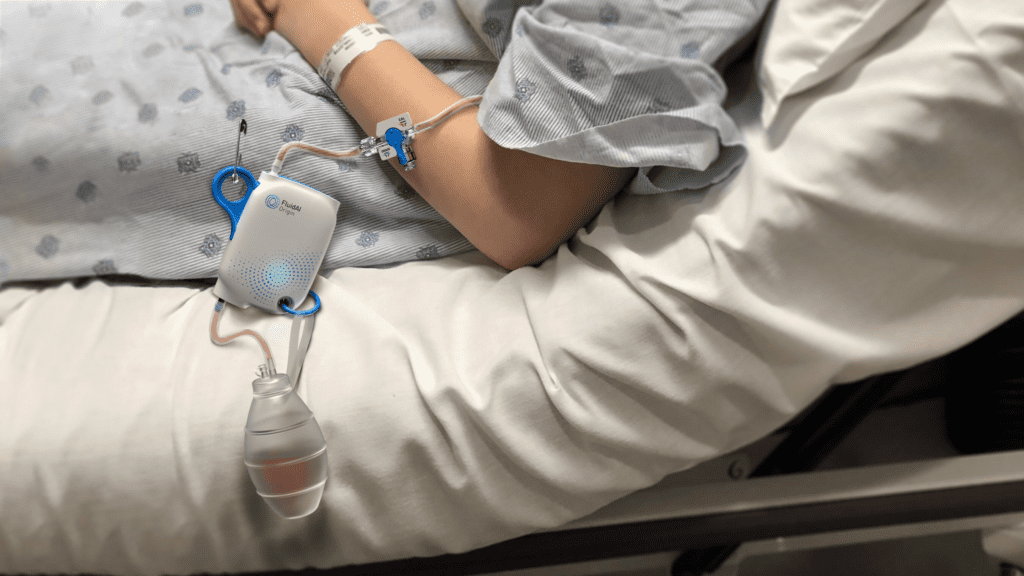
Read MoreFluidAI’s artificial intelligence-powered postsurgical monitor, Stream™ Platform, launches globally
Canadian medical technology company, FluidAI Medical (FluidAI), is set to revolutionize postoperative care with the launch of its ground-breaking medical device, the Stream™ Platform in Canada and Saudi Arabia. Through the use of the Stream™ Platform, surgeons have the potential to make a more accurate and timely diagnosis of postoperative leaks, dreaded complications of digestive tract surgeries, which can lead…
Read MoreGrand Piano Passion Identifies Best Hearing Aid Technology for Music
Grand Piano Passion™, the leading resource for musicians and music lovers with hearing loss, reviews hearing aids and alternative devices for music in their new technology guide. Access the Guide: Hearing Aids and Music Technology. Half of classical musicians and one-third of rock musicians have hearing loss, according to a Hearing Research study, while music lovers…
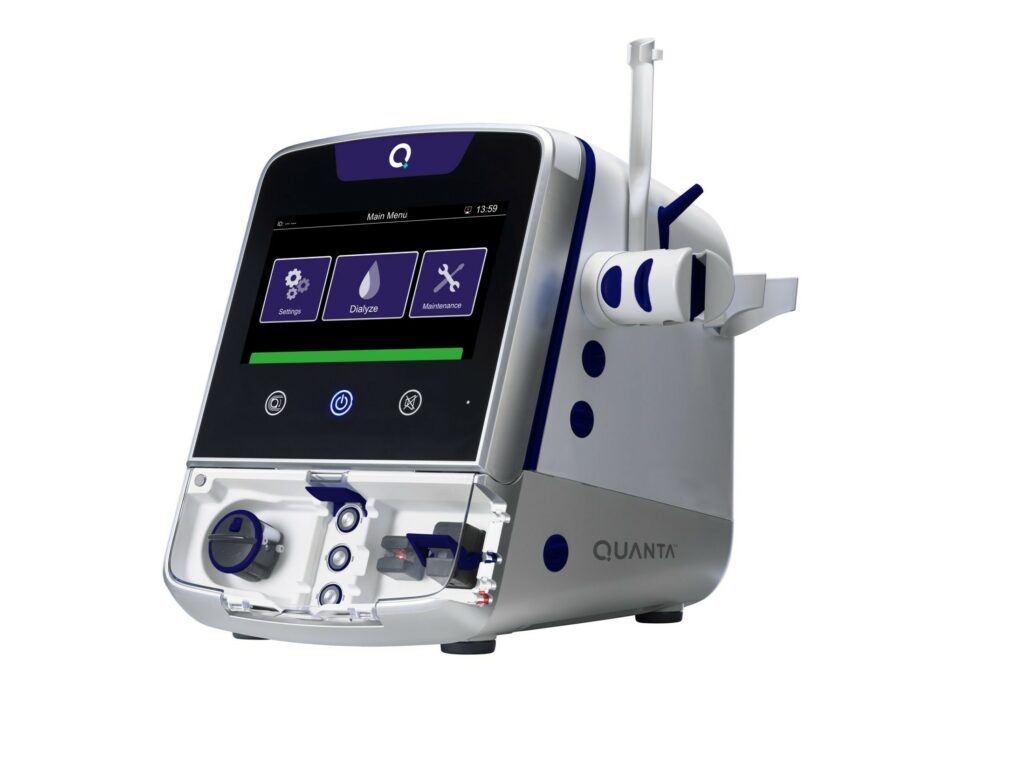
Read MoreQuanta™ Receives FDA 510(k) Clearance for Expanded Indication of Continuous Renal Replacement Therapies
Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for an expanded indication of the Quanta Dialysis System, a compact and easy-to-use hemodialysis device, for two modalities of continuous renal replacement therapy (CRRT): continuous venovenous hemodialysis (CVVHD)…
Read More