Archive for January 2020
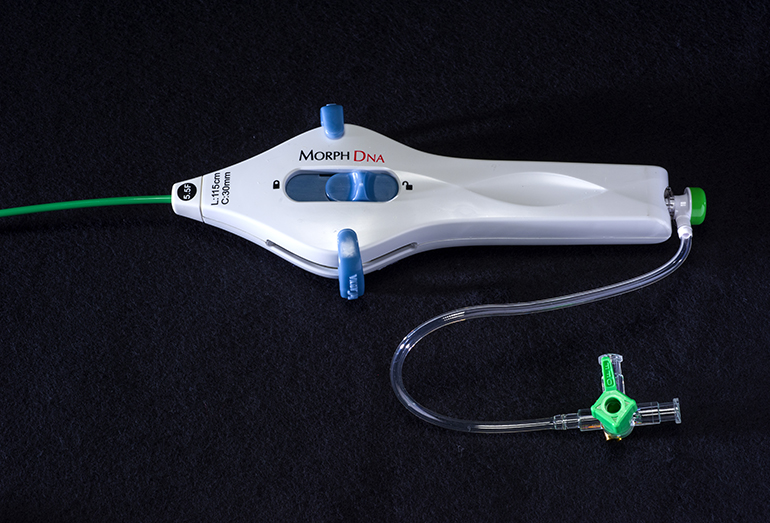
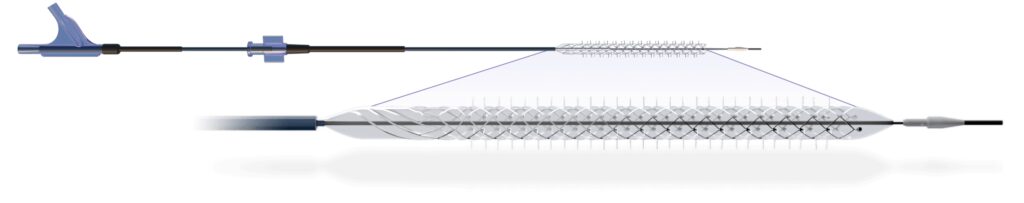
BioCardia Announces FDA Clearance for Morph DNA Catheter to Guide Cell Delivery into Heart Tissue
BioCardia (BCDA)®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Morph® DNA deflectable guide catheter used to guide the Helix™ Biotherapeutic Delivery System during CardiAMP™ cell therapy delivery in the heart. The Morph DNA deflectable…
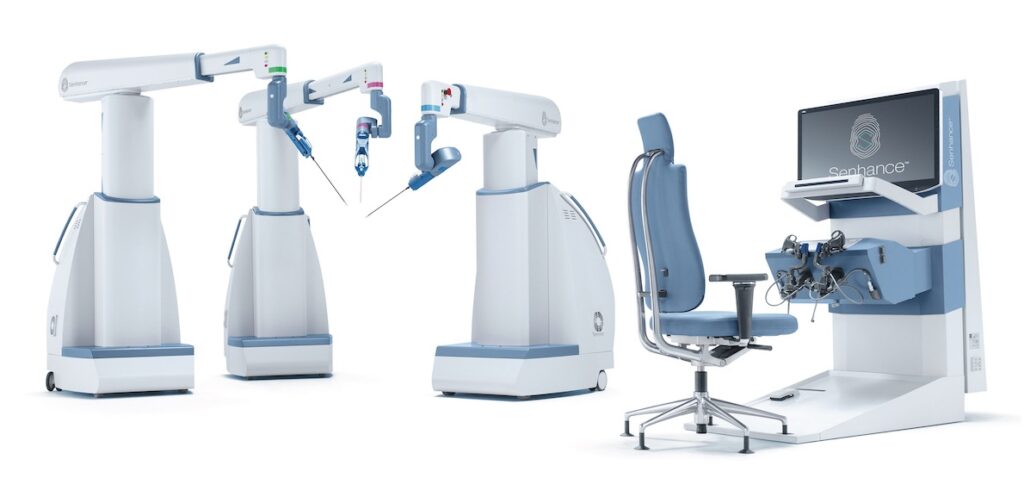
Read MoreTransEnterix Announces Submission of 510(k) to FDA for First Machine Vision System in Robotic Surgery
TransEnterix, Inc. (NYSE American: TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced the Company filed a 510(k) submission with an Intelligent Surgical Unit (ISUTM)1 that is designed to enable machine vision capabilities on the SenhanceⓇ Surgical System. “TransEnterix is the first company to…
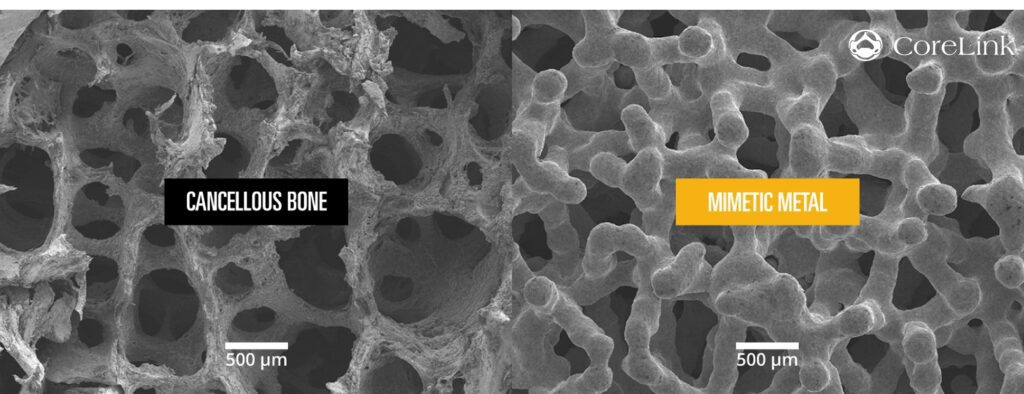
Read MoreCoreLink Surpasses 5,000 Implants With Proprietary 3D Printed Technology
CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the implantation of over 5,000 3D printed devices using their proprietary Mimetic Metal® technology. Mimetic Metal is an additively manufactured technology that combines a lattice framework and inner trabecular pores to emulate the structural, functional and physiological properties of bone. The unique dynamic design…
Read MoreMachine Keeps Livers Alive for a Week, Revives Injured Ones
Researchers from the UniversityHospital Zurich, ETH Zurich, Wyss Zurich and the University of Zurich have developed a machine that repairs injured human livers and keeps them alive outside the body for one week. This breakthrough may increase the number of available organs for transplantation, saving many lives of patients with severe liver diseases or cancer.…
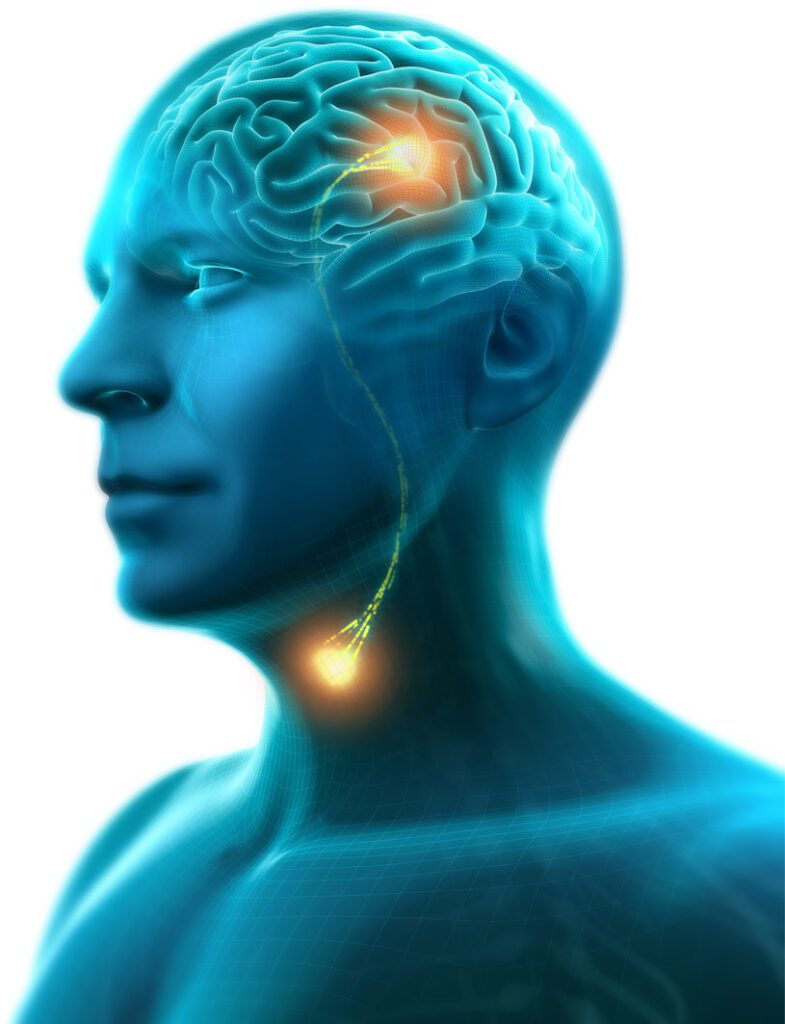
Read MorePhagenesis Wins FDA Breakthrough Nod for Phagenyx System for Restoring Swallowing
Phagenesis Ltd., a pioneering leader in the treatment of dysphagia, is pleased to announce today that the company has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Phagenyx System. The Phagenyx System is a novel neurostimulation device that helps to restore neurological swallowing control through Pharyngeal Electrical Stimulation. The…
Read MoreAI Saving Brain: FDA Clears Aidoc’s Complete AI Stroke Package
Aidoc, the leading provider of AI solutions for radiologists, today announced that the US Food and Drug Administration (FDA) has cleared its AI solution for flagging Large-Vessel Occlusion (LVO) in head CTA scans, marking Aidoc’s fourth FDA-cleared AI package. Combined with Aidoc’s previously-cleared AI module for flagging and prioritizing intracranial hemorrhage, together they provide a comprehensive AI package…
Read MoreColospan Wins IDE FDA Approval for Intraluminal Bypass Device
Colospan which develops novel solutions for colorectal surgery, announced today that the United States Food and Drug Administration (FDA) has approved the company’s investigational device exemption (IDE) application. With this IDE approval in hand, the company will launch its pivotal study for CG-100, a temporary Intraluminal Bypass Device, designed to reduce diverting stoma rates in patients…
Read MoreFDA Grants Breakthrough Device Designation to Reflow Medical’s Temporary Spur Stent System
Reflow Medical announces that the Temporary Spur Stent System, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral artery disease, has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA). The Breakthrough Devices Program is designed to give patients and health care providers timely access…
Read MoreVirtual Incision Announces $20 Million Financing to Advance First-of-its-Kind Surgical Mini-Robot
Virtual Incision Corporation, a medical device company pioneering a first-of-its-kind miniaturized surgical robot, announced it has raised $20 million in a Series B+ financing led by returning investor Bluestem Capital, with participation from returning investor PrairieGold Venture Partners, as well as from Genesis Innovation Group and other affiliated investors. The funds will support regulatory and…
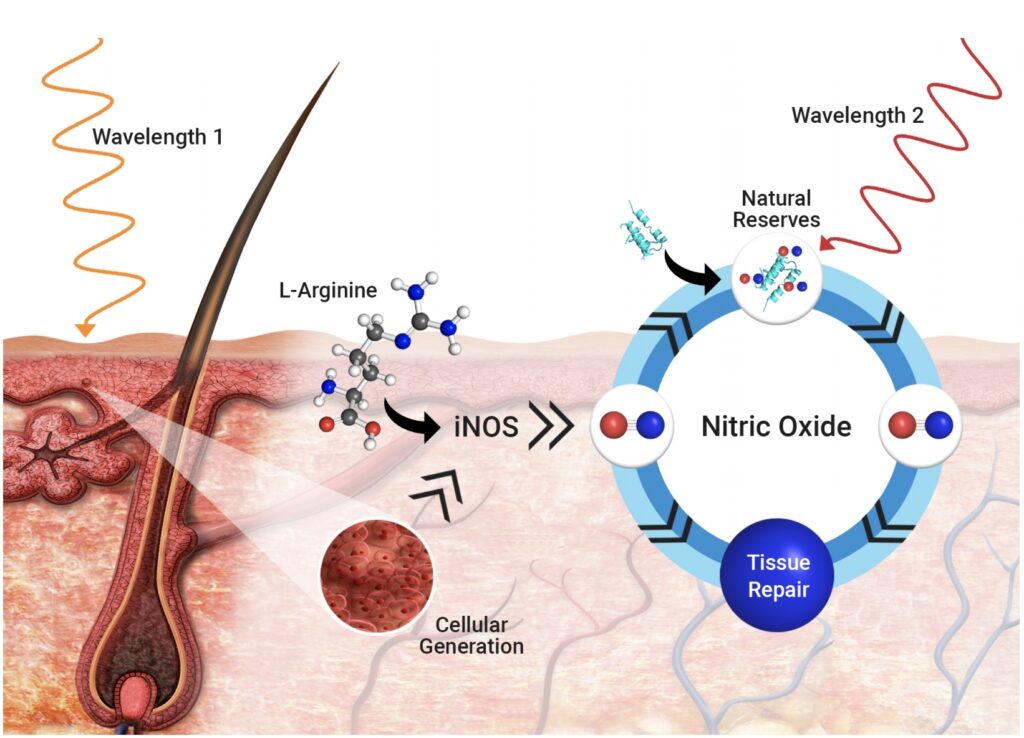
Read MoreREVIAN Inc. Announces Issuance of Landmark Patent Covering the Use of Light to Stimulate the Body to Heal Itself
REVIAN Inc. (formerly PhotonMD Inc.), a medical technology company dedicated to stimulating the body’s natural processes to rejuvenate hair and skin with light, is pleased to announce that the United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 10,265,258 – the seminal patent on the use of proprietary color combinations of light to…
Read More