Archive for January 2020
Medtronic Warns of Mazor X Detachment Issue in Robotic Surgery
Users of Medtronic’s Mazor X Surgical System may experience a hardware detachment problem in which a system piece unexpectedly releases from the OR table, according to an urgent field safety notice issued by the company in December. Medtronic said it had received seven complaints of the issue occurring as of Nov. 13, none of which involved patient…
Read MoreIntralink-Spine, Inc: Announces Continuation of CLBP Clinical Study
Intralink-Spine, Inc. (ILS) announces that it has received unanimous approval from the Data Safety Monitoring Board (DSMB) to proceed with the Réjuve® clinical safety study (GEM-SE) for chronic low back pain (CLBP). As a result, ILS will be adding new sites to broaden Principal Investigator (PI) and participant representation in Australia. “After careful review of…
Read MoreZipThaw, the World’s First Portable, Precise Plasma Thawing Medical Device, Receives FDA Clearance for Clinical Use
FreMon Scientific is pleased to announce the FDA has now cleared ZipThaw™ for frozen plasma thawing as a Class II medical device. ZipThaw, used with the ZipSleeve™ anti-contaminant disposable barrier, is the world’s first dry and portable precision plasma thawing system. Clinicians can now deliver vital plasma to patients at the right time and temperature.…
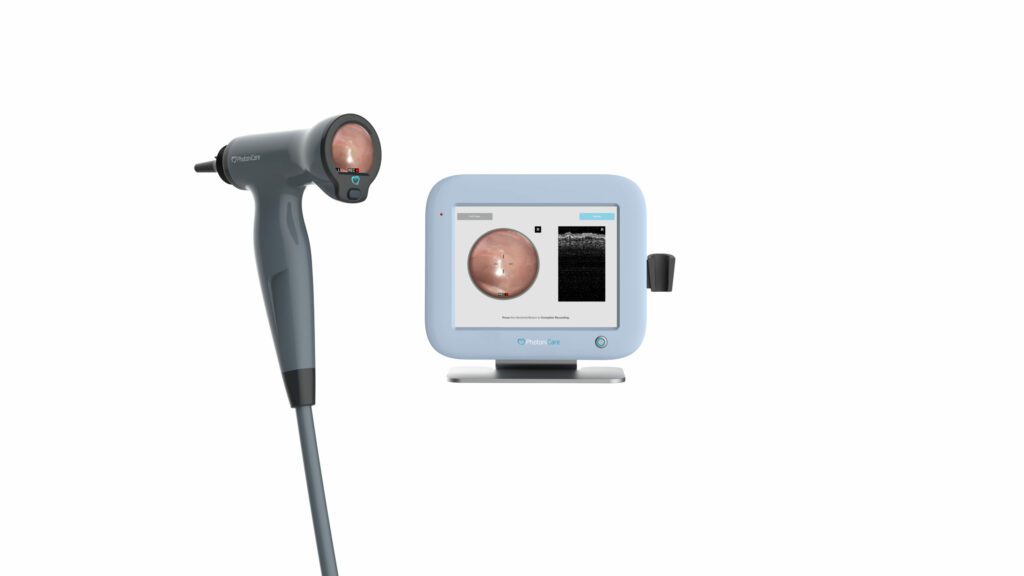
Read MorePhotoniCare Announces FDA Clearance for First-In-Class Technology for Imaging the Ear
PhotoniCare, Inc., a company dedicated to revolutionizing healthcare by providing physicians with better diagnostic tools, today announced that the U.S. Food & Drug Administration (FDA) has granted 510(k) clearance for its TOMi™ Scope for non-invasive imaging of the middle ear. Using optical coherence tomography (OCT) high resolution depth imaging, TOMi Scope helps to determine the presence or absence…
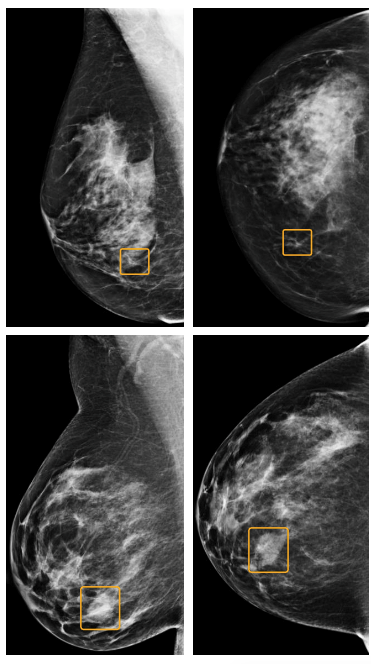
Read MoreGoogle AI Tool Bests Clinicians in Breast Cancer Detection Study
A Google artificial intelligence tool may predict breast cancer better than doctors, according to a study published Wednesday in the journal Nature. The algorithm, which was trained on almost 91,000 mammogram scans from women in the U.S. and U.K., resulted in almost 6% fewer incorrect cancer diagnoses and 9% fewer false negatives than standard clinical practice when…
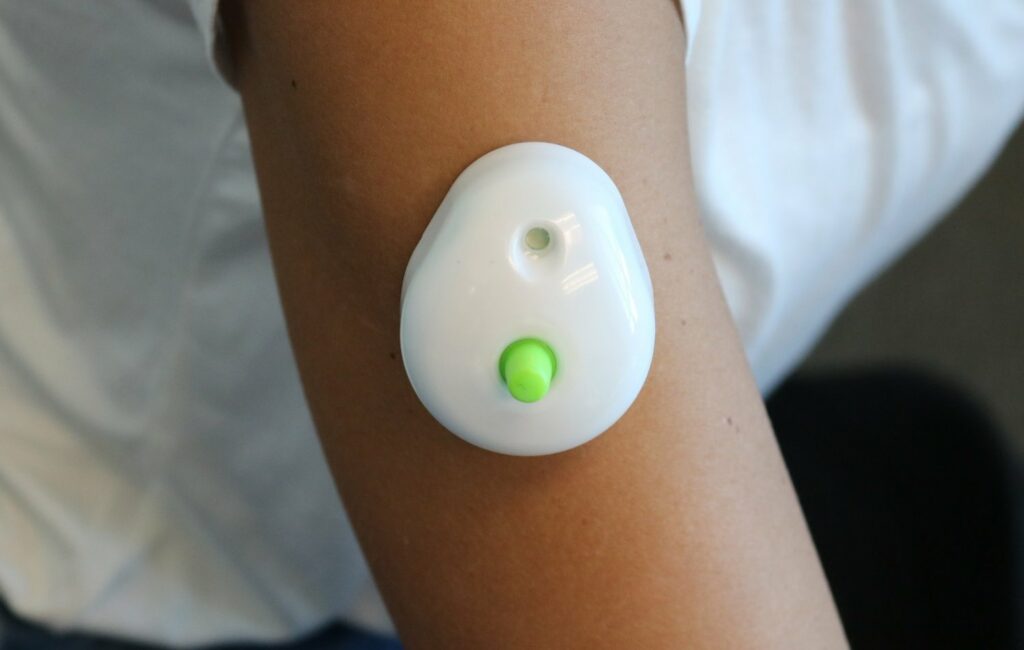
Read MoreTAP At-Home Blood Collection System Now FDA Cleared
Seventh Sense Biosystems, Inc. (7SBio) announced today that the company has received U.S. Food and Drug Administration (FDA) 510(k) authorization to extend its existing clearance to include blood collection by laypersons. Regulators are also allowing the device to be used ‘at-home’ for wellness testing. “We’re very excited about this clearance since it represents an unprecedented…
Read MoreInterscope Announces New FDA Clearance of the EndoRotor, for Use in Airway Procedures Including in Interventional Pulmonology
Interscope, Inc. announced today the receipt of marketing clearance from the FDA for the EndoRotor® System to commercialize Pulmonary indications. Interscope innovated the first flexible microdebrider for use by medical specialists in the digestive tract with reported results reducing the need for surgery in recurrent adenoma and removal of walled off necrosis. The company now looks…
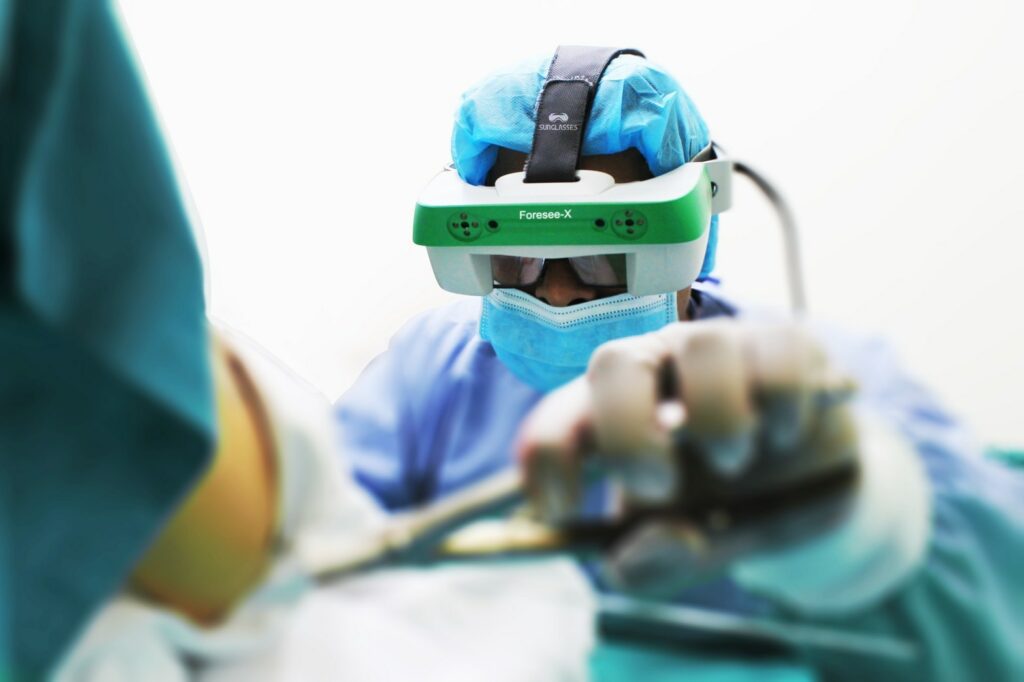
Read MoreForesee-X Augment Reality Solution from SURGLASSES Is Registered with the FDA and Ready for Trauma Treatment
Foresee-X is a set of smart surgical glasses with functionality based on augmented and mixed reality technologies. This device was developed by SURGLASSES, also known as Taiwan Main Orthopaedic Biotechnology Co. Ltd., and has received IEC60601-1-2 and ISO 13485 certifications. It is designed to bring a higher level of support to surgical procedures for trauma cases.…
Read More