Archive for June 2018
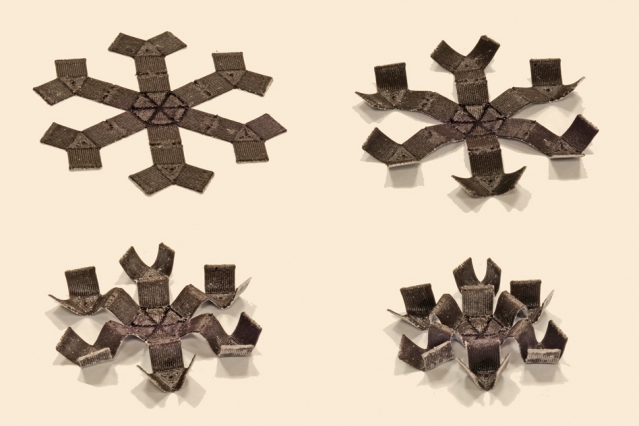
MIT’s 3D Printed Walking, Drug-Transporting Magnets
MIT engineers have created soft, 3-D-printed structures whose movements can be controlled with a wave of a magnet, much like marionettes without the strings. The menagerie of structures that can be magnetically manipulated includes a smooth ring that wrinkles up, a long tube that squeezes shut, a sheet that folds itself, and a spider-like “grabber”…
Read MoreDoctors Told Not to Order ECG’s for Low-Risk Patients
Doctors shouldn’t routinely perform electrocardiograms on patients at low risk for heart disease, an influential federal panel is recommending. While an ECG test of the heart’s electrical activity is safe and inexpensive, the benefits for patients at low risk of heart disease are very low and the results can trigger possibly dangerous, unnecessary follow-up testing…
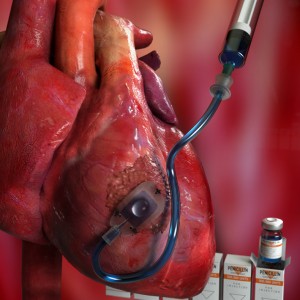
Read MoreImplantable Device Delivers Drugs Straight to the Heart
An international team of researchers, led by Harvard University, have developed a refillable, implantable device, which sits directly on the heart and can deliver drugs and other therapies to treat the aftereffects of a heart attack. The device, dubbed Therepi, is a small patch that is sutured onto the heart. The patch contains a sponge-like…
Read MoreIntuitive Surgical Invests $15m Into Lung Cancer Startup
Intuitive Surgical has struck a deal with lung disease technology firm Broncus Medical. The multipart agreement includes a $15 million investment, technology development collaboration and a licensing deal. Broncus has built its business upon a portfolio of technology platforms that support the diagnosis of lung cancer and treatment of emphysema. These devices provide image guidance to help…
Read MorePositive Clinical Data for Penny-Sized Bioelectronic Implant
SetPoint Medical’s open-label study supports the use of the company’s bioelectronic therapy for Crohn’s Disease. The Valencia,CA-based company’s study was conducted at five European sites and included 16 patients with moderate to severe Crohn’s Disease with inadequate responses to tumor necrosis factor (TNF) antagonist drugs and other non-TNF targeted biologic agents. The data, presented…
Read MoreBoston Scientific Will Not Comment on Reported Stryker Takeover Offer
Boston Scientific said Monday it won’t comment on reports rival medical device manufacturer Stryker made a takeover bid, which was first reported by The Wall Street Journal. Stryker’s shares tumbled more than 4 percent, and Boston Scientific’s rose nearly 8 percent Monday in afternoon trading. They were halted before Boston released its statement. “Boston Scientific … is aware…
Read MoreStryker Makes Takeover Approach to Boston Scientific
Boston Scientific shares rose nearly 7 percent Monday after the Wall Street Journal reported rival medical device manufacturer Stryker approached it with a takeover bid. A deal would combine two medical device giants into a powerhouse with a combined value of more than $110 billion, The Wall Street Journal reported, citing people familiar with the matter. Sources told…
Read MoreInsightec’s MR-Guided Focused Ultrasound Wins Medicare Coverage in 10 States
INSIGHTEC®, a global medical technology innovator of incisionless surgery, today announced that Medicare benefit coverage is available to patients in 10 U.S. states for MR-guided focused ultrasound (MRgFUS) for the treatment of essential tremor (ET). Six more states will follow on July 1, 2018. Additional Medicare Administrative Contractors (MAC) have issued positive Draft Local Coverage Determination…
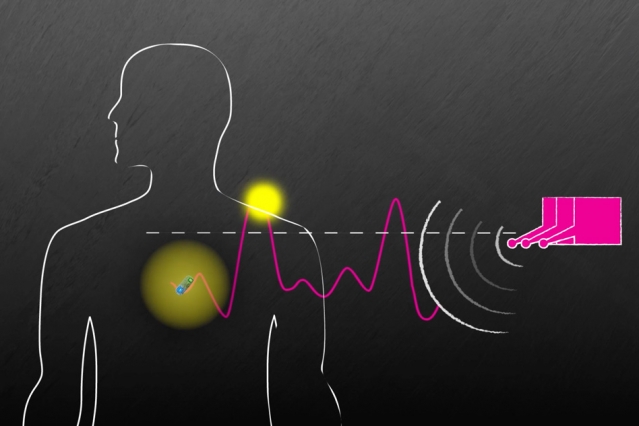
Read MoreMIT Team Creates Wireless System to Power Implanted Devices
Researchers from the Massachusetts Institute of Technology (MIT) have collaborated with local Brigham and Women’s Hospital to create a new system that can wirelessly power and communicate with medical devices inside the body. The new In Vivo Networking (IVN) system employs radio frequency waves to power implanted devices. This is expected to eliminate the need…
Read More5 new deaths from liquid-filled intragastric balloons prompt FDA warning
The FDA today released a notice warning of five new patient deaths related to two liquid-filled intragastric balloon systems used to treat obesity, produced by Apollo Endosurgery (NSDQ:APEN) and ReShape Lifesciences (NSDQ:RSLS). The additional deaths bring the total number of deaths worldwide from the Orbera intragastric balloon and ReShape integrated dual balloon system up to 12 since 2016, the…
Read More