Archive for April 2020
Sterilucent Granted Emergency Use Authorization to Reprocess Respirators
The U.S. Food and Drug Administration has granted Sterilucent, Inc. (Minneapolis, MN), an Emergency Use Authorization to allow the emergency use of the Sterilucent™ HC 80TT Vaporized Hydrogen Peroxide Sterilizer for decontaminating single-use compatible N95 and N95-equivalent respirators. Test results have demonstrated that filtering facepiece respirators may be reprocessed for use during the COVID-19 pandemic…
Read MoreOlympus Launches EVIS X1, its Most Advanced Endoscopy System to Date
Olympus Corporation (President: Yasuo Takeuchi) today announced the launch of EVIS X1, its most advanced endoscopy system to date. The new system is to improve outcomes from disorders of the stomach, colon, and oesophagus, as well as from bronchial diseases, by providing every endoscopist with innovative and proven tools. The launch of EVIS X1 further…
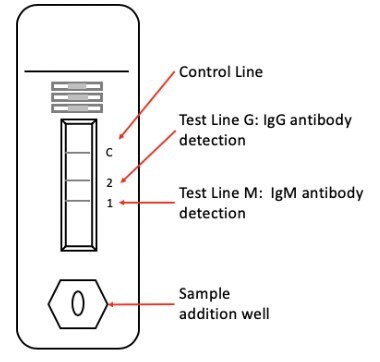
Read MoreZEUS Scientific Announces New Rapid SARS-CoV-2 Antibody Test
ZEUS Scientific announces today the submission for an Emergency Use Authorization (EUA) to the U.S. Food and Drug Administration (FDA) for its rapid, in vitro diagnostic test for the qualitative detection of IgG and/or IgM antibodies to the SARS-CoV-2 (novel 2019 Coronavirus). ZEUS’s lateral flow test uses patient serum, plasma or whole blood and provides results in…
Read MoreNew Renewable Energy Agreements To Reduce Boston Scientific Carbon Footprint By Half
Boston Scientific Corporation (NYSE: BSX) has signed a virtual power purchase agreement (VPPA) that represents the largest step the company has taken to achieve its goal of global carbon neutral manufacturing and distribution by 2030. The agreement, with Clearway Energy Group, will address the electricity load for the company’s U.S. operations, which represents 45 percent of…
Read MoreBrainlab Announces CE Mark for ExacTrac Dynamic Patient Positioning and Monitoring
Brainlab, the digital medical technology company, today announced CE Mark (Conformité Européenne) approval for ExacTrac® Dynamic, the company’s next generation patient positioning and monitoring system. The new system offers never-before-seen, high-speed thermal surface tracking technology combined with an update of ExacTrac X-ray monitoring, providing advanced capabilities. New clinical workflows allow for treatment of a wide array of…
Read MoreVent Multiplexor Receives FDA Emergency Use Authorization for Crisis Care Co-Ventilation During COVID-19 Pandemic
Vent Multiplexor, LLC and Yale New Haven Hospital announced today that the Food and Drug Administration has granted Emergency Use Authorization for the Vent Multiplexor, a life-saving emergency rescue device developed by Vent Multiplexor LLC in collaboration with Yale New Haven Hospital. The Vent Multiplexor is a patent-pending device designed to provide individualized emergency crisis…
Read MoreFDA Grants NEXUS Aortic Arch Stent Graft System Breakthrough Designation
Endospan, a pioneer in off-the-shelf endovascular repair of aortic arch disease was recently granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the NEXUS™ Aortic Arch Stent Graft System. The FDA’s Breakthrough Device Designation Program is intended to provide timely access to medical devices that have the potential to provide a…
Read MoreFDA Encourages Recovered Patients to Donate Plasma for Development of Blood-Related Therapies
As part of the all-of-America approach to fighting the COVID-19 pandemic, the U.S. Food and Drug Administration has been working with partners across the U.S. government, academia and industry to expedite the development and availability of critical medical products to treat this novel virus. Today, we are providing an update on one potential treatment called…
Read MoreCentinel Spine Announces FDA Approval for Two-level prodisc L Total Disc Replacement
Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced FDA approval of two-level indications for the prodisc® L Lumbar Total Disc Replacement (TDR) system. Centinel Spine now becomes the only company in the world with an FDA-approved lumbar TDR device that has been clinically reviewed and found safe and effective for…
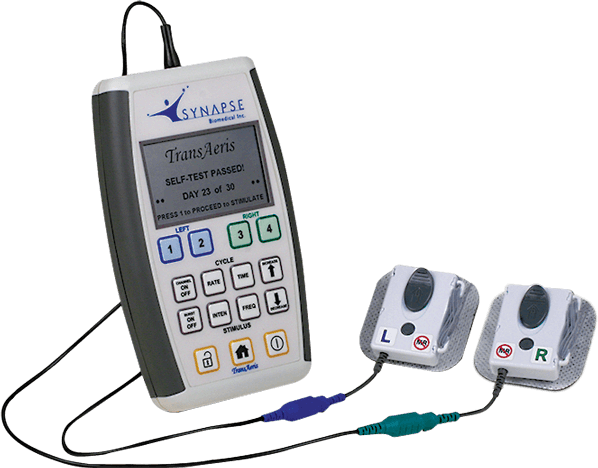
Read MoreSynapse Biomedical’s TransAeris Diaphragm Pacing System Gets FDA Emergency Use Authorization for Quicker Ventilator Weaning
Synapse Biomedical, Inc. has received an Emergency Use Authorization for the emergency use of its TransAeris® DPS, to assist in weaning patients determined by their healthcare provider to be at high risk of weaning failure off of ventilators in healthcare settings during the COVID-19 pandemic for no more than 30 days. During the COVID-19 pandemic, the…
Read More