Archive for April 2020
New Study Finds HealthySole UVC Technology Deactivates COVID-19 on Footwear
Researchers have proven a device using ultraviolet light technology can neutralize the COVID-19 virus and other infectious diseases on the soles of shoes by more than 99.5 percent, according to a new study. The device, called HealthySole® PLUS, is being introduced in hospitals and other settings where infection control is urgent. A home version of the…
Read MoreAdvanced Sterilization Products Granted FDA EUA Allowing Decontamination of Millions of Compatible N95 Respirators to Help Protect Against COVID-19
Advanced Sterilization Products (ASP), a Fortive (NYSE:FTV) company and global leader in infection prevention, has received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for the use of STERRAD® Systems to decontaminate compatible N95 respirators.1 Utilizing equipment already available onsite in many US hospitals, STERRAD Systems could collectively reprocess millions of compatible N95 respirators daily.…
Read MoreFDA Clears New Disposable Fog-Free Articulating 5mm Laparoscope
Xenocor, Inc. announced the FDA cleared the new Xenocor Disposable 5mm Articulating Laparoscope for minimally invasive abdominal and thoracic surgery. The Xenoscope 5mm articulating disposable laparoscope is designed to improve image quality, reduce fog, lower hospital costs, and reduce bio-hazard risk for the patient and staff. “Disposable laparoscopes can reduce hospital costs and prevent cross-contamination between…
Read MoreTack Endovascular System Receives FDA Approval for Below-the-Knee Post-Angioplasty Dissection Repair
Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced it received U.S. Food and Drug Administration (FDA) approval for the Tack Endovascular System (4F), a novel, minimal metal implant for precision dissection repair in the mid/distal popliteal, tibial and/or peroneal arteries. Regulatory approval was based on data from…
Read MoreVomaris Seeking Expedited FDA Approval for New Virus-Killing Face Mask
Vomaris Innovations, Inc., headquartered in Tempe, AZ, announced today that significant research findings confirmed viruses are killed upon exposure to the company’s bioelectric V.Dox™ Technology platform. Vomaris has begun applying their technology to manufacture prototype face masks that can be worn alone or inside an existing face mask with the intention of killing viruses and reducing…
Read MoreAbbott’s TriClip Becomes First Device of its Kind to Receive CE Mark for Minimally Invasive Tricuspid Valve Repair
Abbott announced that its TriClip™ Transcatheter Tricuspid Valve Repair System has received CE Mark and is now approved for use in Europe and other countries that recognize CE Mark as a non-surgical treatment for people with a leaky tricuspid valve, a condition known as tricuspid regurgitation (TR). With the CE Mark designation, Abbott’s TriClip device is…
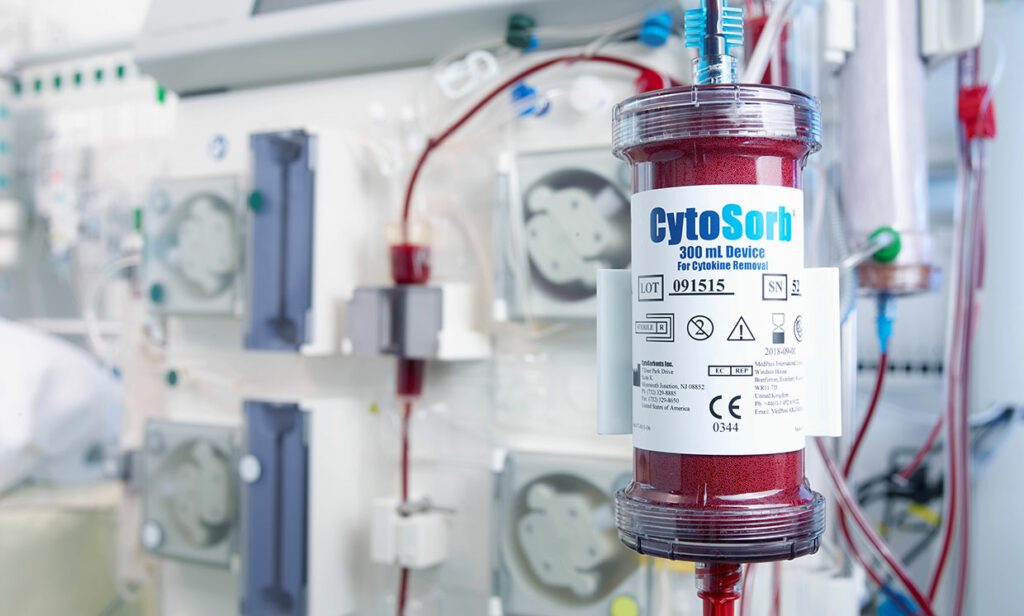
Read MoreU.S. FDA Grants CytoSorb Emergency Use Authorization for Use in Patients with COVID-19 Infection
CytoSorbents Corporation, a critical care immunotherapy leader commercializing its CytoSorb® blood purification technology to treat cytokine storm and deadly inflammation in critically-ill and cardiac surgery patients around the world, announced the United States Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) of CytoSorb® for use in patients with COVID-19 infection. Under the EUA, CytoSorbents can make CytoSorb available, through…
Read MoreFDA Grants Breakthrough Device Designation to DNAe’s Sequencing Diagnostic
DNAe, the next generation sequencing company developing novel diagnostics for use at the point-of-need, today announced that the US Food and Drug Administration (FDA) has granted it a “Breakthrough Device” designation for its pioneering platform and first assay. DNAe has reinvented sequencing in order to develop a compact device operable by non-specialist users. LiDia-SEQ™ will,…
Read MoreSpiderTech Helping Healthcare Workers Experiencing Irritation, Sores From PPE With Face Protection
In the midst of the COVID-19 pandemic, healthcare workers across the world have been active on social media displaying the painful results of wearing medical personal protective equipment (PPE) for long hours on the job. Face masks, surgical and N95 masks, eyewear, facial hoods and other protective measures have resulted in red, sore, irritated skin…
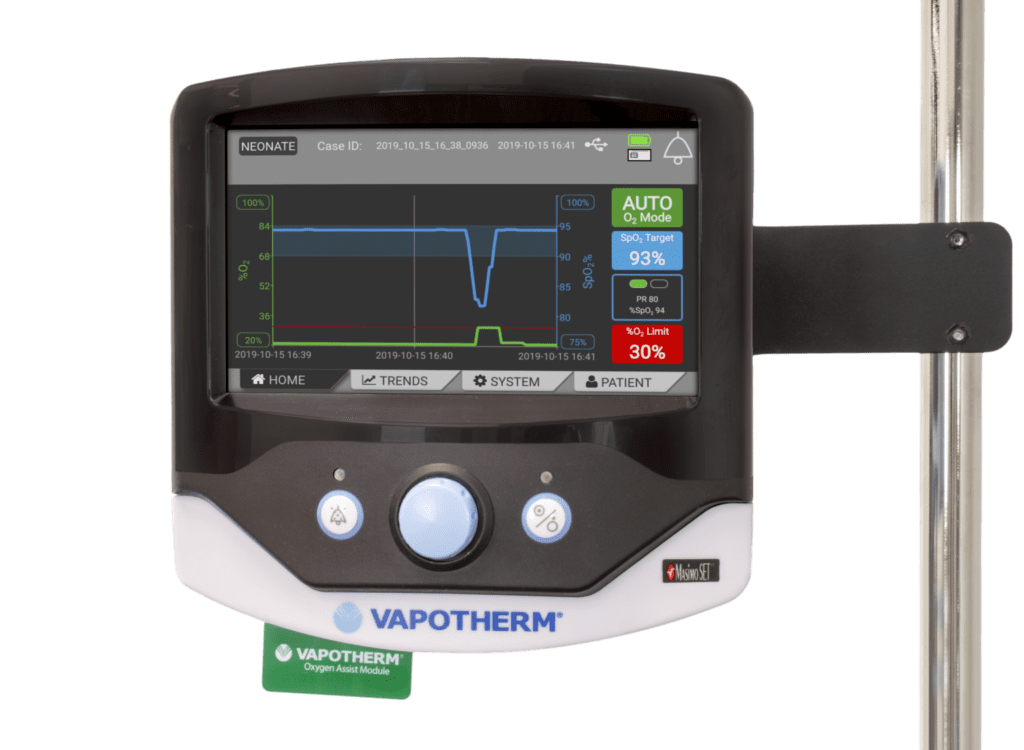
Read MoreFDA Grants Vapotherm Oxygen Assist Module (OAM) Breakthrough Device Designation
Vapotherm, Inc., a global medical technology company focused on the development and commercialization of its proprietary Hi-VNI® Technology products that are used to treat patients of all ages suffering from respiratory distress, today announced that the U.S. Food and Drug Administration (FDA) recently granted Breakthrough Device Designation for the Company’s Oxygen Assist Module (OAM). FDA’s…
Read More