Archive for August 2018
Avista to acquire Organogenesis
Avista Healthcare Public Acquisition Corp., a publicly traded special purpose acquisition company, and Organogenesis Inc., a leading regenerative medicine company focused on the development, manufacture and commercialization of product solutions for the Advanced Wound Care, Surgical and Sports Medicine markets, today announced that they have entered into a definitive merger agreement, under which Organogenesis will…
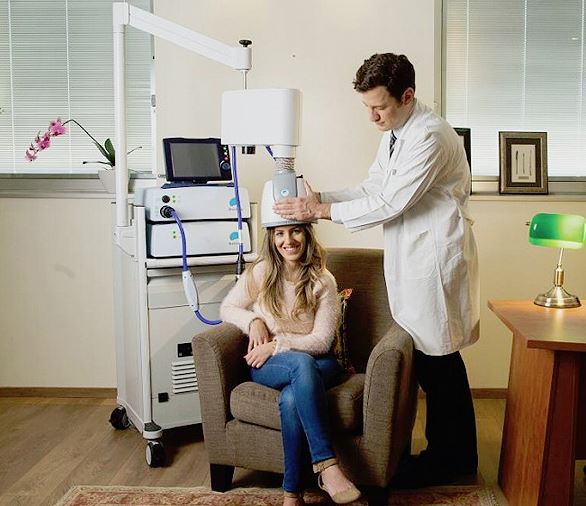
Read MoreFDA Clears Brainsway TMS System to Treat OCD
On Friday, the FDA said it granted de novo approval for Brainsway‘s deep transcranial magnetic stimulation system, now indicated for treating obsessive compulsive disorder. The TMS system uses magnetic fields to simulate nerves in the brain, and has been shown to reduce the severity of OCD in patients. A 100-patient randomized, multi-center study of the device…
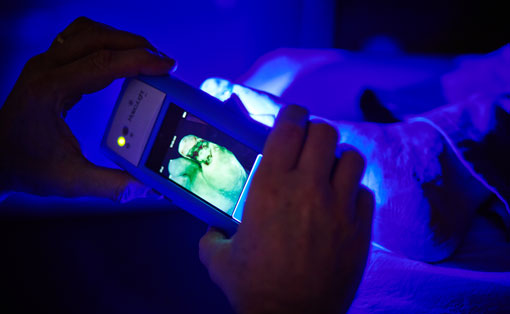
Read MoreMolecuLight’s First-of-its-kind Handheld Wound Imaging Device Receives FDA De Novo Clearance
MolecuLight Inc. achieved a major regulatory milestone permitting expansion into the United States market. FDA has granted De Novo clearance for the ground-breaking wound fluorescence imaging device, the MolecuLight i:X. The device digitally captures and documents fluorescence information from wounds and surrounding tissue using still images and videos in real-time. This product is optimized for use at the point-of-care. It…
Read MoreBioSig’s High-Fidelity Electrophysiology System Approved by FDA
BioSig Technologies, Inc. announced that the Company has received 510(k) clearance for its first product, PURE EP System, from the U.S. Food and Drug Administration (FDA). The non-invasive PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients…
Read More7D Surgical’s Cranial Machine-Vision Guided Surgical System FDA Approved
7D Surgical announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Cranial Module. This achievement is a crucial step in the commercial launch of 7D Surgical’s innovative Machine-vision Image Guided Surgery (MvIGS) system for cranial surgery throughout the United States. The 7D Surgical System utilizes completely…
Read MoreSiemens & Nuvasive Partner on Spinal Surgery Workflow Development
NuVasive said last week it had inked a strategic partnership deal with Siemens Healthineers looking to develop and advance technology designed for minimally invasive spinal surgery. Through the deal, dubbed the Spine Precision Partnership, the companies will look to jointly advance their proprietary technologies, looking to improve operating room workflow efficiency and the delivery of minimally disruptive spinal…
Read MoreBoston Scientific Spends $160M on Veniti’s Venous Stents
Boston Scientific is proving itself to have more stamina than the Energizer Bunny when it comes to making acquisitions. The Marlborough, MA-based company is now picking up Veniti, a firm that has developed a stent system for treating venous obstructive disease. The deal consists of $108 million up-front cash as well as up to $52 million in…
Read MoreWorld’s Smallest Clot Retriever for Strokes Receives CE Mark
Rapid Medical, based in Yokneam, Israel, won European regulatory approval to introduce its TIGERTRIEVER 13, the narrowest clot retriever now available for use in treating ischemic stroke. The device can be used in vessels as small as 1 millimeter in width and up to a maximum of 2.5 mm, where larger retrievers would be appropriate. It works…
Read MoreOrange County to Use First CT Scanner for Autopsies in Florida
Florida’s Orange County is investing half a million dollars in a CT scanner for its local morgue, a move expected to speed up the autopsy process and facilitate criminal investigations without cutting into victims’ bodies, WFTV reported this week. The CT machine county officials have their eyes on is one currently being used in fewer…
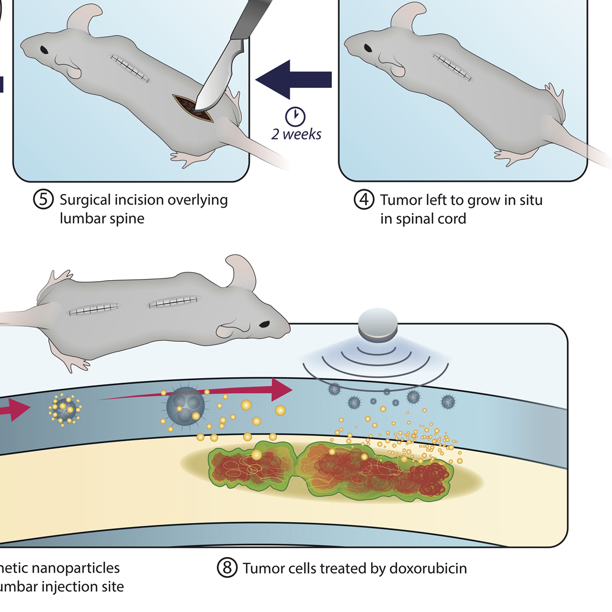
Read MoreMagnetic Surgical Cement Pulls Chemotherapy Drugs across Blood-Brain Barrier into Spinal Tumors
Patients with spinal fractures caused by tumors or osteoporosis usually undergo a procedure called kyphoplasty, where the fracture is filled with surgical cement. While kyphoplasty can stabilize the bone, cancer patients are still often left with spinal column tumors that are very hard to reach with conventional chemotherapy, which has to cross the blood-brain barrier…
Read More