Archive for July 2023
FDA Clears ReddyPort® Non-Invasive Ventilation Device
ReddyPort announced today that the US Food and Drug Administration (FDA) granted 510(k) premarket clearance for the ReddyPort elbow device used in non-invasive ventilation (NIV). “ReddyPort’s patented elbow is central to the eco-system we are building to help mitigate clinical obstacles tied to NIV therapy from dry-mouth, oral biofilm accumulation to speech recognition,” says Tony Lair,…
Read MoreWillowWood Launches Fiberglass Meta® Shock X
Building on the success of META® Shock X, WillowWood launches the Fiberglass META® Shock X. The revolutionary META® feet combine responsive energy return with balance, stability, and impact protection. All META® feet feature the industry’s first unibody platform, free of hardware for a minimal and lightweight, but durable design. Fiberglass META Shock® X features our…
Read MoreAllosource Receives FDA 510(K) Clearance for Aceconnex™ Pre-Sutured Fascia for Hip Labral Reconstruction and Augmentation
AlloSource®, one of the largest allograft providers creating innovative cellular and tissue products to help surgeons heal their patients, today announced the U.S. Food and Drug Administration’s 510(K) clearance of AceConnex Pre-Sutured Fascia for hip labral reconstruction and augmentation. This product reinforces AlloSource’s commitment to providing innovative products to support the overall sports medicine market,…
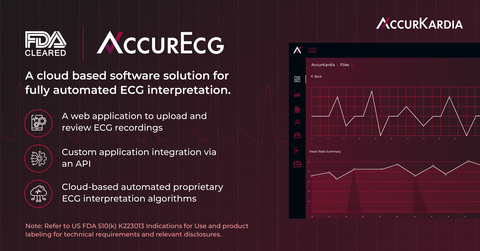
Read MoreAccurKardia’s AccurECG™ Analysis System Receives FDA 510(k) Clearance
AccurKardia, a medical technology company delivering clinical-grade ECG interpretation software, announced today that its flagship product, the AccurECG™ Analysis System (“AccurECG” or the “System”), has been granted FDA 510(k) clearance. AccurECG™ is a cloud-based, device-agnostic and fully automated electrocardiogram (ECG) interpretation software platform. The groundbreaking AccurECG™ software provides an array of benefits, such as beat-by-beat analysis, ventricular/supraventricular…
Read MoreInvictus Announces FDA Clearance of Ground-Breaking Neoasis® ANC Device
Invictus Medical announced today that the culmination of their De Novo application to the FDA for its Neoasis® incubator-based active noise control (ANC) device has resulted in a clearance-for-use declaration by the FDA. “With this clearance for use, Invictus has made a huge step towards deploying the Neoasis® ANC device in neonatal intensive care units. It is well documented…
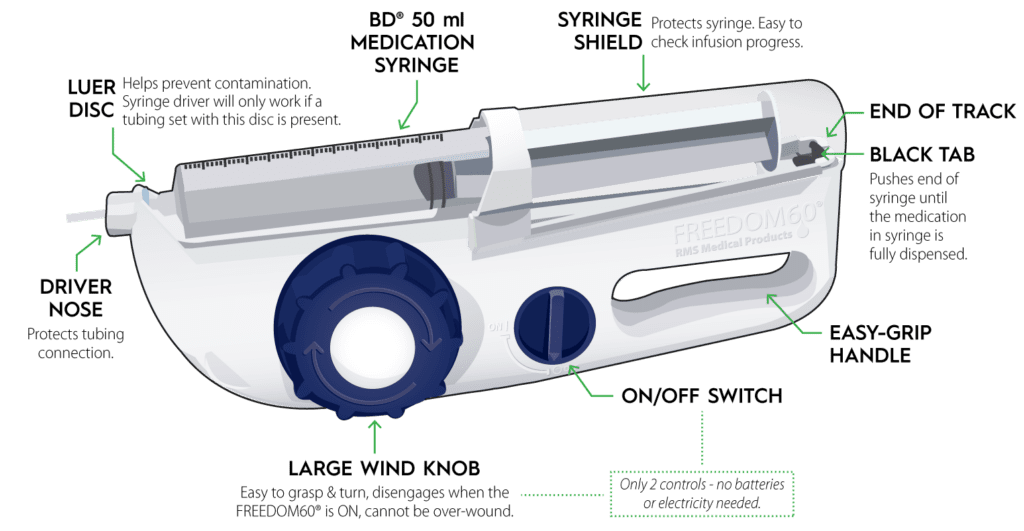
Read MoreKORU Medical Systems, Inc. Announces 510(K) Submission for Freedom60® Infusion System With Hizentra® 50 Ml Prefilled Syringes
KORU Medical Systems, Inc. (NASDAQ: KRMD) (“KORU Medical” or the “Company”), a leading medical technology company focused on the development, manufacturing, and commercialization of innovative and easy-to-use specialty subcutaneous infusion solutions that improve quality of life for patients, today announced that it has submitted a 510(k) premarket notification submission to the U.S. Food and Drug…
Read MoreColoplast Announces Agreement to Acquire Kerecis and Raises Long-term Growth Expectations
Coloplast has signed an agreement to acquire Kerecis, an innovative, fast-growing company in the biologics wound care segment, for up to USD 1.3 billion (around DKK 8.9 billion), of which USD 1.2 billion (around DKK 8.2 billion) is an upfront cash payment See Full Press Release at the Source: Coloplast announces agreement to acquire Kerecis and…
Read MoreMedRhythms Announces FDA Listing of InTandem™ (MR-001) to Improve Walking and Ambulation in Adults with Chronic Stroke
MedRhythms announced that MR-001, the company’s evidence-based neurorehabilitation system to improve walking and ambulation in adults with chronic stroke walking deficits, has been listed as a Class II medical device with the U.S. Food and Drug Administration (FDA). The system delivers an intervention based on the principle of Rhythmic Auditory Stimulation (RAS), a well-researched clinical…
Read More