Orthopedics and Spine
PathKeeper Surgical Commercializes Spine Navigation System with First U.S. Cases
PathKeeper Surgical, a privately-held, Israel-based, medical technology company, dedicated to improving the health of individuals around the world suffering from all spinal issues requiring surgery, while making navigation-guided surgery available to any patient in all operating rooms, announces the first commercial use of the PathKeeper, 3D optical navigation system in the United States. The surgical debut of the…
Read MoreConformis Announces Definitive Agreement to be Acquired by restor3d for a Purchase Price of $2.27 Per Share in Cash
restor3d, Inc. and Conformis, Inc. (NASDAQ: CFMS) announced today that they have entered into a definitive merger agreement under which restor3d, a leading personalized 3D-printed orthopedic company, will acquire all outstanding shares of common stock of Conformis at $2.27 per share in cash, which represents an approximate 96 percent premium to the closing price of…
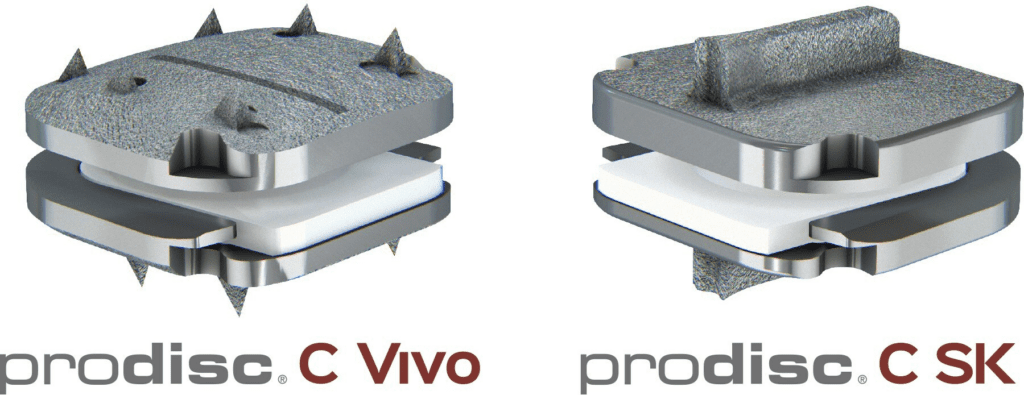
Read MoreCentinel Spine® Completes Enrollment in First-of-its-Kind 2-Level IDE Trial Evaluating prodisc® C Match-the-Disc™ Cervical TDR System
Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced the completion of enrollment in a first-of-its-kind Investigational Device Exemption (IDE) study evaluating the Company’s prodisc C Vivo and prodisc C…
Read MoreArtelon, Inc.’s FlexBand®, FlexPatch®, and FlexBand Plus® earn FDA Clearance for Ligament Reinforcement
Artelon Inc., announced today U.S. FDA 510(k) clearance of FlexBand®, FlexPatch®, and FlexBand Plus® for ligament repair surgery in addition to tendon repairs*. This new clearance expands the indications for these products to now include reinforcement of medial, lateral and ulnar collateral ligaments, spring ligaments, deltoid ligaments, and extra-articular ligaments in the ankle, knee, and…
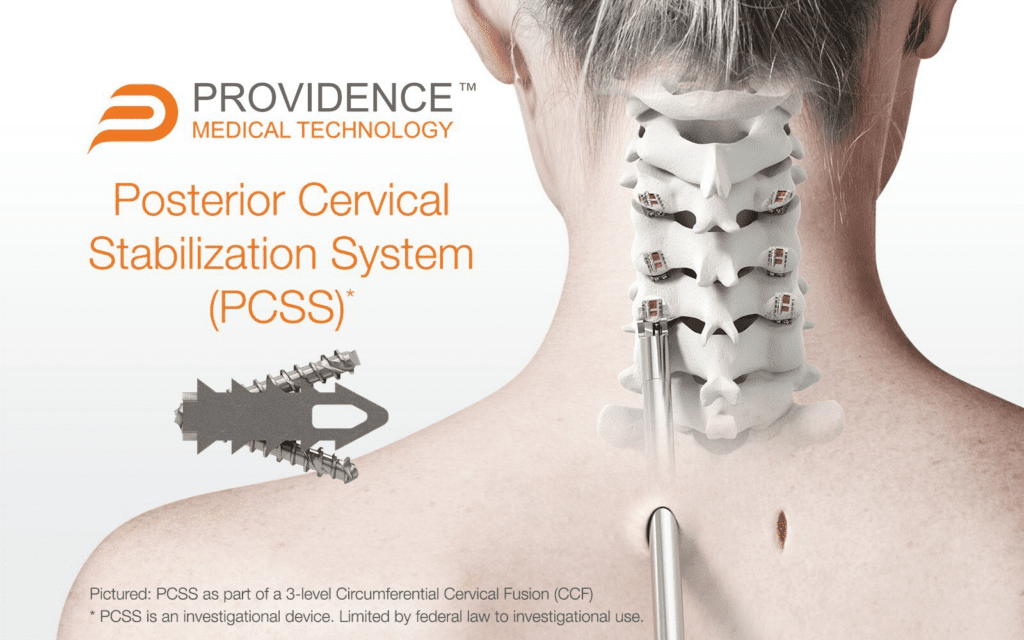
Read MoreProvidence Medical Technology Announces Completion of Enrollment in the FUSE Clinical Study for High-Risk Cervical Fusion Patients
Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the close of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients. The study enrolled…
Read MoreAbyrx Receives Additional FDA Clearances for MONTAGE® Settable, Resorbable Bone Putty as Bone Void Filler and Cranial Bone Cement
Abyrx, Inc. (http://abyrx.com), a leading biomaterial sciences company with a focus on therapeutic technologies for use during surgical procedures, today announced its receipt of additional United States Food and Drug Administration (FDA) clearances for the company’s MONTAGE® Settable, Resorbable Bone Putty as both a bone void filler and cranial bone cement. Used by surgeons across…
Read MoreFDA Authorizes Marketing of MISHA™ Knee System for People Suffering from Knee Osteoarthritis
Moximed, a medical device company on a mission to improve the standard of care for people with knee osteoarthritis (OA), today announced that the U.S. Food and Drug Administration (FDA) granted marketing authorization of the MISHA™ Knee System, an implantable shock absorber (ISA) for the knee. The MISHA Knee System is indicated to treat people…
Read Morerestor3d Receives FDA Clearance of Patient Specific Resection Guides for use with the Kinos Axiom Total Ankle System
restor3d, a research-driven medical device company, announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to the Axiom PSR System for use with the company’s Kinos Axiom Total Ankle System. The Axiom PSR (patient-specific resection) System is additively manufactured from titanium alloy, making it the first all-metal, patient-specific instrument system cleared…
Read MoreX-Bolt Orthopedics Announces FDA 510(k) Clearance for Pro-X1™ Trochanteric Nail
X-Bolt, an emerging innovator of orthopedic devices, is pleased to announce that its hip fracture solution, the Pro-X1 Trochanteric Nail, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pro-X1 will be X-Bolt’s flagship launch in the United States. “We are thrilled to receive FDA clearance for the Pro-X1 Trochanteric Nail,” said Brian Thornes,…
Read MoreShoulder Innovations Announces Oversubscribed $42 million Series D Financing
Shoulder Innovations Inc. (SI), a Grand Rapids, Michigan based leader in the shoulder replacement implant market, announced closing on an oversubscribed $42 million Series D financing. SI is a privately-held medical device company with a broad team of successful innovators in the shoulder space. The financing was led by Gilde Healthcare Partners, a specialized trans-Atlantic healthcare investor. US Venture Partners, Lightstone Ventures,…
Read More