Orthopedics and Spine
CurvaFix Launches Smaller-diameter CurvaFix® IM Implant
CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, announced the launch of its smaller-diameter, 7.5mm CurvaFix® IM Implant, designed to simplify surgery and provide strong, stable fixation in small-boned patients. The company will showcase the new 7.5mm intramedullary device, of which over two dozen have already been implanted in patients, at…
Read MoreCatalyst Receives FDA 510(k) Clearance for its Convertible Stemmed Total Shoulder Arthroplasty System
Catalyst OrthoScience Inc. (Catalyst), a medical device company focused on the upper extremity orthopedics market, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its fully convertible stemmed total shoulder arthroplasty system with an ellipsoid anatomic head. This is the market’s first stemmed arthroplasty system featuring an ellipsoid humeral head,…
Read MoreGlobus Medical and NuVasive to Combine in All-Stock Transaction to Create Innovative Global Musculoskeletal Company Focused on Patient Care
Globus Medical (NYSE: GMED), a leading musculoskeletal solutions company, and NUVASIVE (NASDAQ: NUVA), the leader in spine technology innovation, today announced they have entered into a definitive agreement to combine in an all-stock transaction. The transaction brings together two well-regarded technology companies in the musculoskeletal industry, which have a shared vision focused on innovation in…
Read MoreTyber Medical LLC Acquires ADSM-Synchro Medical
Tyber Medical LLC has acquired 100 percent of the capital of ADSM-Synchro Medical, a French orthopedic medical device company, specializing in the development and distribution of implants dedicated to treating surgical forefoot pathologies. Founded in 2010, ADSM has become a market leader in the European medical device industry with products like the ToeGrip Classic, the…
Read MoreConventus Flower Orthopedics Announces Expansion of its Flex-Thread™ Technology Platform with FDA Clearance of the Ulna Intramedullary Nail System
Conventus Flower Orthopedics, an innovation-driven medical technology company, announced they have received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Flex-Thread™ Ulna Intramedullary (IM) Nail System. The Flex-Thread Ulna Nail marks a new application and portfolio expansion to the company’s Flex-Thread technology platform. Expected to launch in February 2023, the Flex-Thread Ulna IM Nail…
Read MoreHip Innovation Technology Announces Initiation of FDA Approved Investigational Device Exemption Study
Hip Innovation Technology, LLC (HIT), a medical device company developing innovative orthopaedic device solutions to advance the quality of life and quality of care for patients, is pleased to announce the first U.S. implantation of the HIT Reverse Hip Replacement System (Reverse HRS), under an FDA approved Investigational Device Exemption (IDE). The IDE Study is…
Read MoreEmpirical Spine Completes Full Premarket Approval (PMA) Submission to FDA for Limiflex™ Dynamic Sagittal Tether™
Empirical Spine, Inc., a medical device company creating a new class of spinal implant, recently completed the final step in the U.S. Food & Drug Administration (FDA) submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year results from the pivotal Investigational Device…
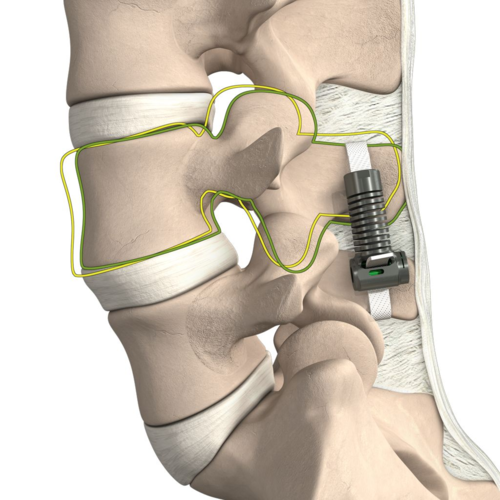
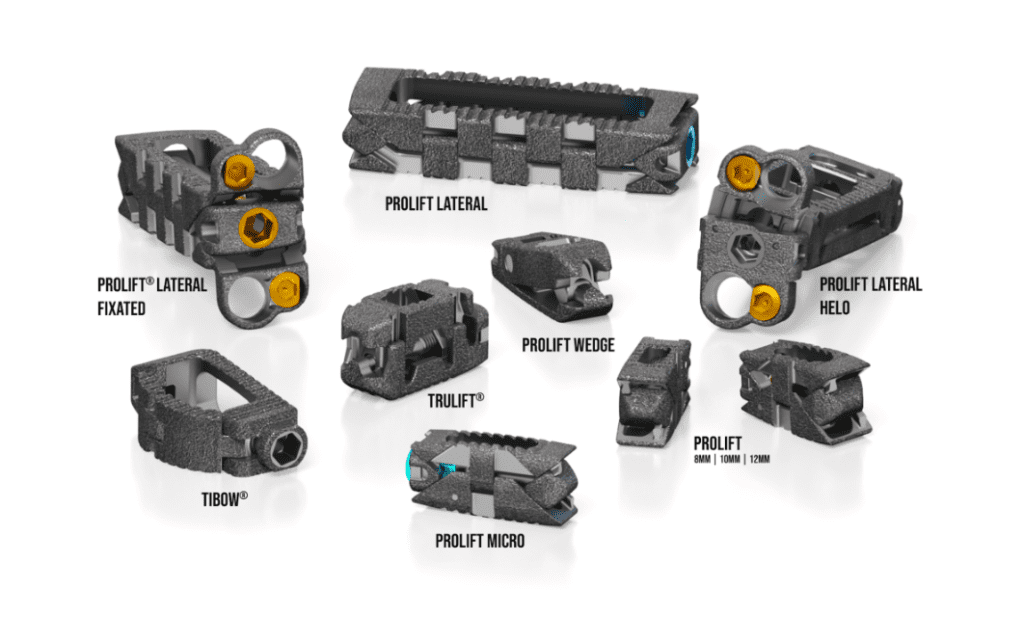
Read MoreLife Spine Announces FDA 510(K) Clearance for the TruLift® Lateral Expandable Spacer System and Lateral Plate System
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the TruLift Lateral Expandable Spacer System and Lateral Plate System. Utilizing expandable technology and robust plating options, TruLift Lateral…
Read MoreChoiceSpine™ Announces Line Extension of their Harrier™ Stand-Alone Anterior Lumbar Interbody Fusion System
ChoiceSpine LLC, a privately-held spinal device manufacturer based in Knoxville, TN, announces the launch of an additional lordotic offering for their stand-alone anterior lumbar interbody – Harrier SA. Harrier SA is a 3D printed stand-alone screw-based system incorporating ChoiceSpine’s proprietary BioBond™ porous structure technology. The system has had great success since launching in May of…
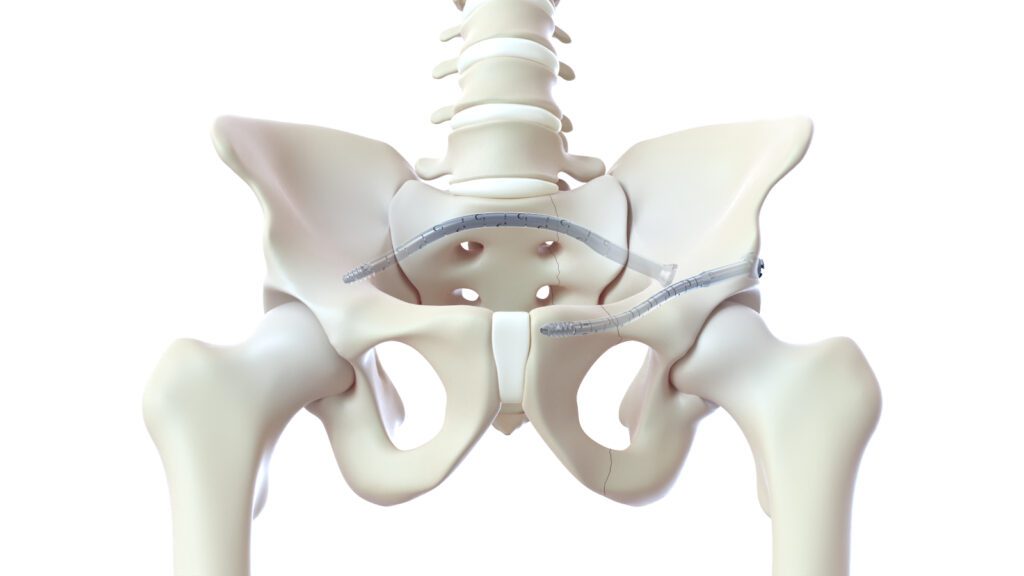
Read MoreCurvaFix Receives FDA Clearance for Smaller-diameter, Intramedullary Implant for Pelvic Fracture Fixation
CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, announced it has received 510(k) clearance (K222505) from the U.S. Food & Drug Administration (FDA) for its smaller-diameter CurvaFix® IM Implant indicated for fixation of fractures of the pelvis. The new 7.5mm device is designed to simplify surgery and to provide strong,…
Read More