Orthopedics and Spine
ulrich Medical USA™ Receives 510(k) Clearance for Flux-C™ 3D Printed Porous Titanium Cervical Interbody Prior to NASS
ulrich medical USA, Inc., a privately held medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, announced the FDA has given 510(k) clearance of its Flux-C 3D printed porous titanium cervical interbody device. “Surgeons have many options for cervical interbodies. The Flux-C porous titanium device offers one of the…
Read MoreOSSIO Announces U.S. Launch and First Commercial Use of OSSIOfiber® Suture Anchors
OSSIO, Inc., a fast-growing orthopedic fixation technology company, announced the U.S. launch and first commercial use of OSSIOfiber® Suture Anchors, expanding patient access to the company’s growing portfolio of bio-integrative implants for use in foot/ankle, shoulder, knee, hand/wrist and elbow surgery. Gregory Berlet, M.D., founding partner at Orthopedic Foot and Ankle Center in Columbus, Ohio, recently…
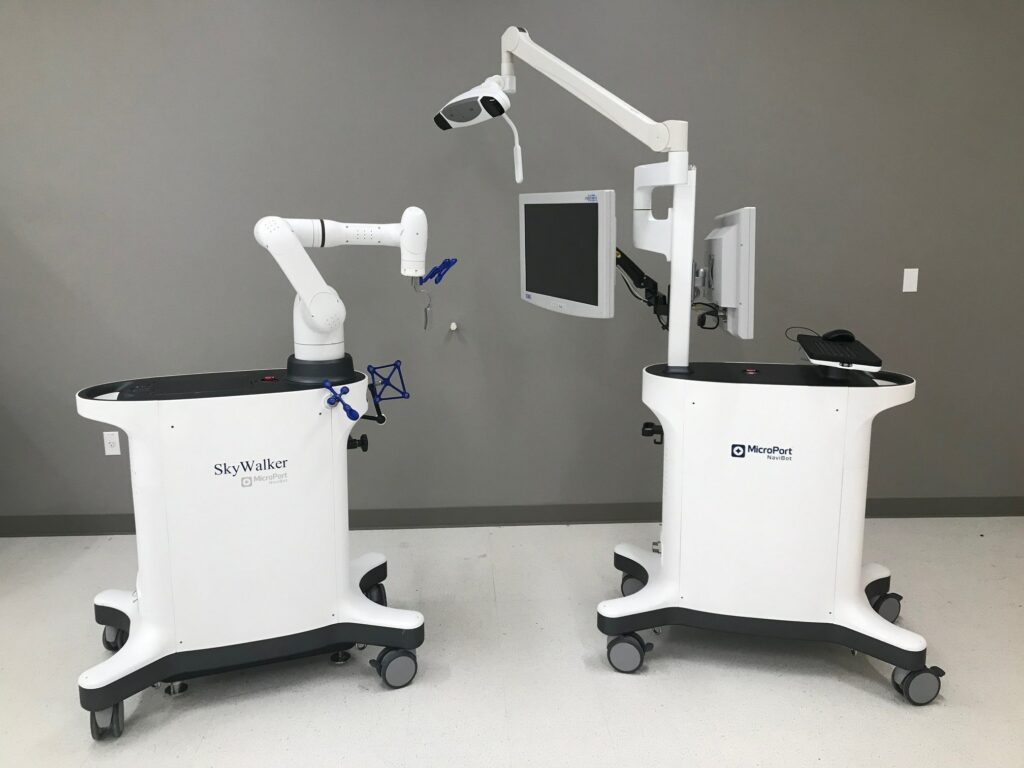
Read MoreMicroPort Navibot Receives 510(K) Clearance for its SkyWalker™ Robot-Assisted Platform for Orthopedic Applications
MicroPort Navibot has received 510(K) clearance from the Food and Drug Administration (FDA) in the United States for the SkyWalker™ System, the company’s first robot-assisted platform for orthopedic applications. The SkyWalker™ System will initially offer a robotically assisted total knee replacement solution that is compatible with the Evolution® Medial-Pivot Total Knee System. Designed based on clinical needs, MicroPort…
Read MoreSpineology® Launches First Fully Endoscopic, Single-Tubular Retractor Fusion System: OptiLIF® Endo
Spineology Inc., the longtime leader in ultra-minimally invasive spine surgery, announces another milestone with the limited launch of OptiLIF® Endo. This innovative, ultra-MIS system requires only one tubular retractor to seamlessly integrate endoscopes and endoscopic equipment into lumbar interbody fusion procedures. The OptiMesh® Multiplanar Expandable Implant enables this single tube system to employ the smallest diameter tubular retractor of…
Read MoreXenco Medical Expands its Ambulatory Surgery Center Device Portfolio with FDA Clearance and Launch of its Multilevel CerviKit
Xenco Medical has expanded its ASC surgical device portfolio through the FDA clearance and launch of its Multilevel CerviKit™, an expansion of Xenco Medical’s breakthrough single-use cervical spine technology to include a comprehensive suite of implants and single-use instruments for 2, 3, and 4 level anterior cervical spine procedures. Optimized for the ambulatory surgery center…
Read MoreExactech Announces FDA Breakthrough Device Designation for JointMedica’s Polymotion® Hip Resurfacing System
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced that the U.S. Food and Drug Administration (FDA) has granted a Breakthrough Device Designation for JointMedica’s Polymotion® Hip Resurfacing System. Exactech, a minority shareholder of JointMedica Limited, is collaborating with the United Kingdom-based orthopaedic device designer and manufacturer to deliver…
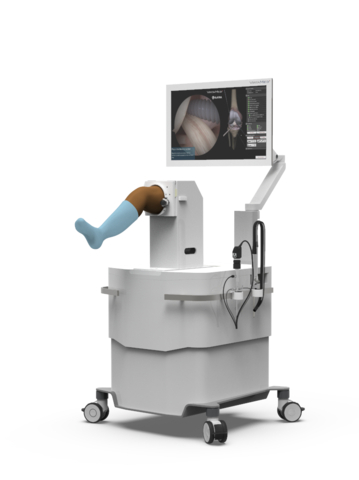
Read MoreVirtaMed Announces New Inclusive Knee Simulation Model
VirtaMed, the world leader in medical simulation training, has announced the release of enhanced simulation training modules for arthroscopic knee surgeries. In a first for VirtaMed, these latest training modules will now be available on simulators with two different skin tones. “It is important to learn medical skills in a realistic environment, and that means…
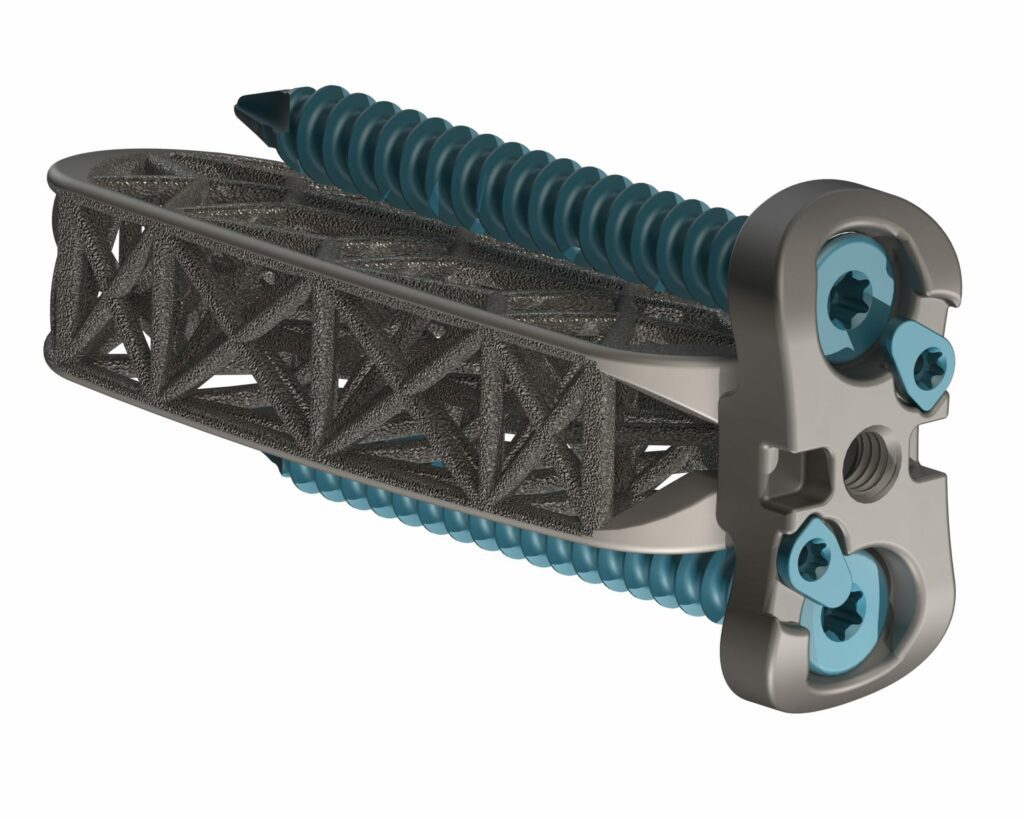
Read More4WEB Medical Launches Hyperlordotic Lateral Implant Portfolio
4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced the company’s launch of a comprehensive array of hyperlordotic lateral implants. The first procedure was performed by Brad Prybis, MD, a surgeon at Tanner Medical Center in Carollton, GA. “The application of 4WEB’s…
Read MoreSpino Modulation Vertebral Body Tethering Device Wins FDA Breakthrough Device Designation
Spino Modulation Inc., a subsidiary of Spinologics Inc., announced today that the U.S. Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for its MIScoli™ system, an innovative vertebral body tethering (VBT) device to treat scoliosis in young adolescents. The FDA Breakthrough Device Program is intended to help patients receive more timely…
Read MoreCerapedics Announces FDA Breakthrough Device Designation Granted for P-15L Bone Graft for the Treatment of Degenerative Disc Disease
Cerapedics Inc., an ortho-biologics company dedicated to enhancing the science of bone repair, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation for its investigational P-15L Bone Graft for the treatment of degenerative disc disease (DDD). The FDA’s Breakthrough Device designation is designed to expedite the development and review of medical devices…
Read More