Archive for April 2023
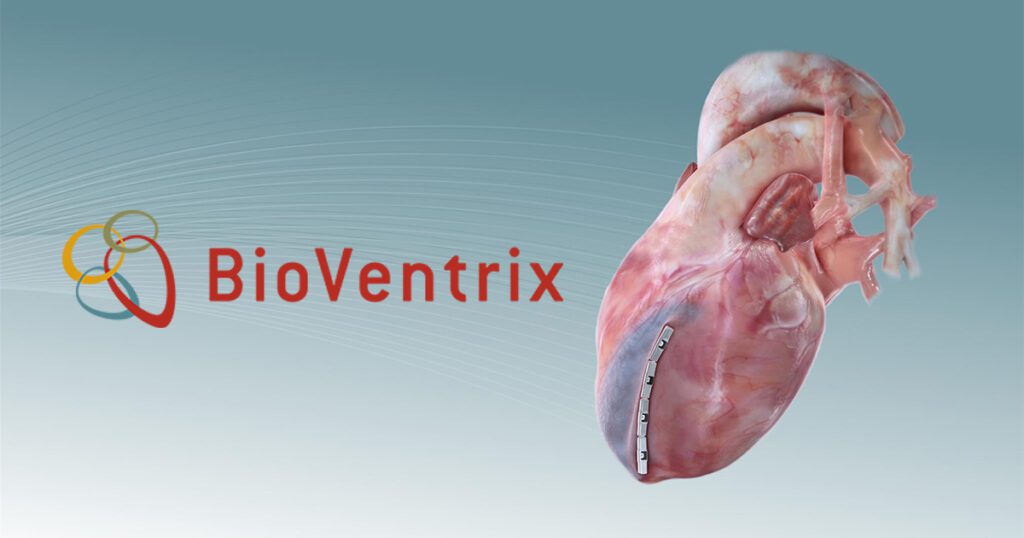
BioVentrix® Closes $48.5 Million Series A Financing
BioVentrix, Inc., a privately held medical device company developing minimally invasive therapies to directly treat the dilated left ventricle and reverse the left ventricular remodeling process in patients with congestive heart failure, today announced the completion of $48.5 million Series A round financing. The round was led by Andera Partners, a prominent life-science venture capital investor. The…
Read MoreAbyrx Receives Additional FDA Clearances for MONTAGE® Settable, Resorbable Bone Putty as Bone Void Filler and Cranial Bone Cement
Abyrx, Inc. (http://abyrx.com), a leading biomaterial sciences company with a focus on therapeutic technologies for use during surgical procedures, today announced its receipt of additional United States Food and Drug Administration (FDA) clearances for the company’s MONTAGE® Settable, Resorbable Bone Putty as both a bone void filler and cranial bone cement. Used by surgeons across…
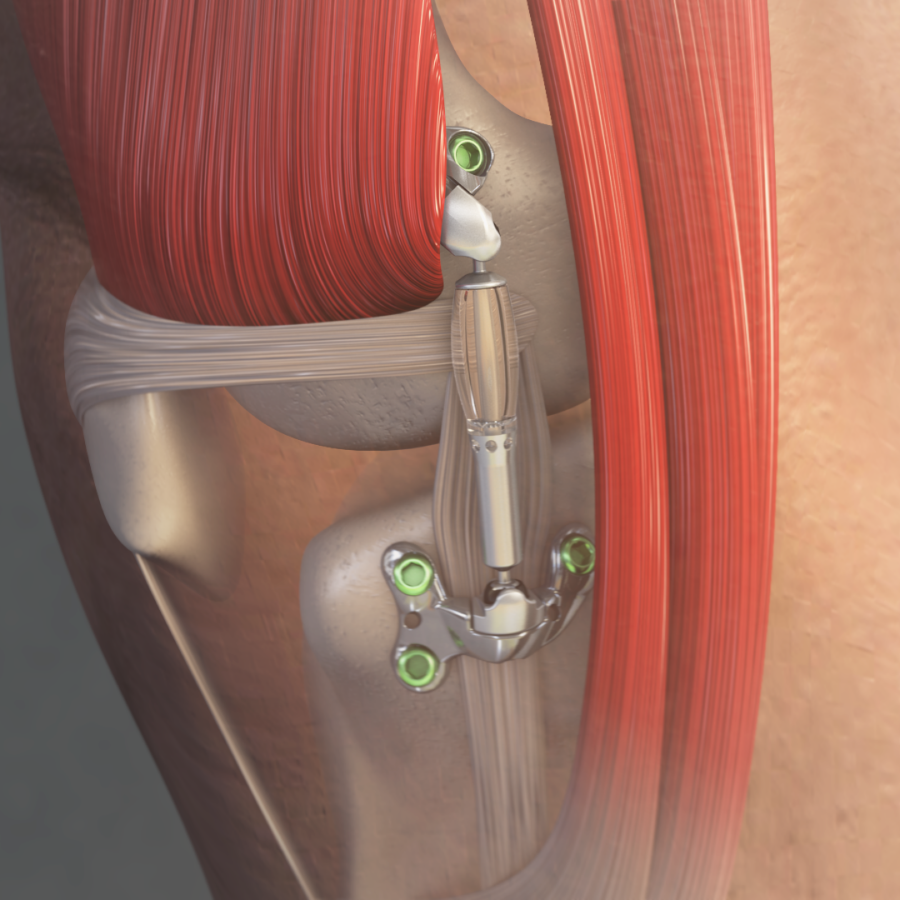
Read MoreFDA Authorizes Marketing of MISHA™ Knee System for People Suffering from Knee Osteoarthritis
Moximed, a medical device company on a mission to improve the standard of care for people with knee osteoarthritis (OA), today announced that the U.S. Food and Drug Administration (FDA) granted marketing authorization of the MISHA™ Knee System, an implantable shock absorber (ISA) for the knee. The MISHA Knee System is indicated to treat people…
Read Morerestor3d Receives FDA Clearance of Patient Specific Resection Guides for use with the Kinos Axiom Total Ankle System
restor3d, a research-driven medical device company, announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to the Axiom PSR System for use with the company’s Kinos Axiom Total Ankle System. The Axiom PSR (patient-specific resection) System is additively manufactured from titanium alloy, making it the first all-metal, patient-specific instrument system cleared…
Read MoreIcentia Receives U.S. Food and Drug Administration (FDA) Clearance for CardioSTAT®
Icentia Inc., announced that it has received FDA 510(k) clearance for CardioSTAT, an ambulatory, continuous ECG monitoring solution that relies on a wire free, single-use recorder. “This approval marks a key milestone for our company. The FDA clearance opens the door to the world’s largest medical device market. With the cost effectiveness and demonstrated ability of…
Read MoreSynapse Biomedical Wins New Approval for Diaphragm Pacing System to Free Patients from Ventilators
Synapse Biomedical, Inc. announced today that the FDA has granted premarket approval (PMA) of the NeuRx® Diaphragm Pacing System (NeuRx DPS®) for use in patients with spinal cord injuries who rely on mechanical ventilation. PMA is the most stringent type of device marketing application required by FDA. More hospitals are expected to begin implementing the NeuRx…
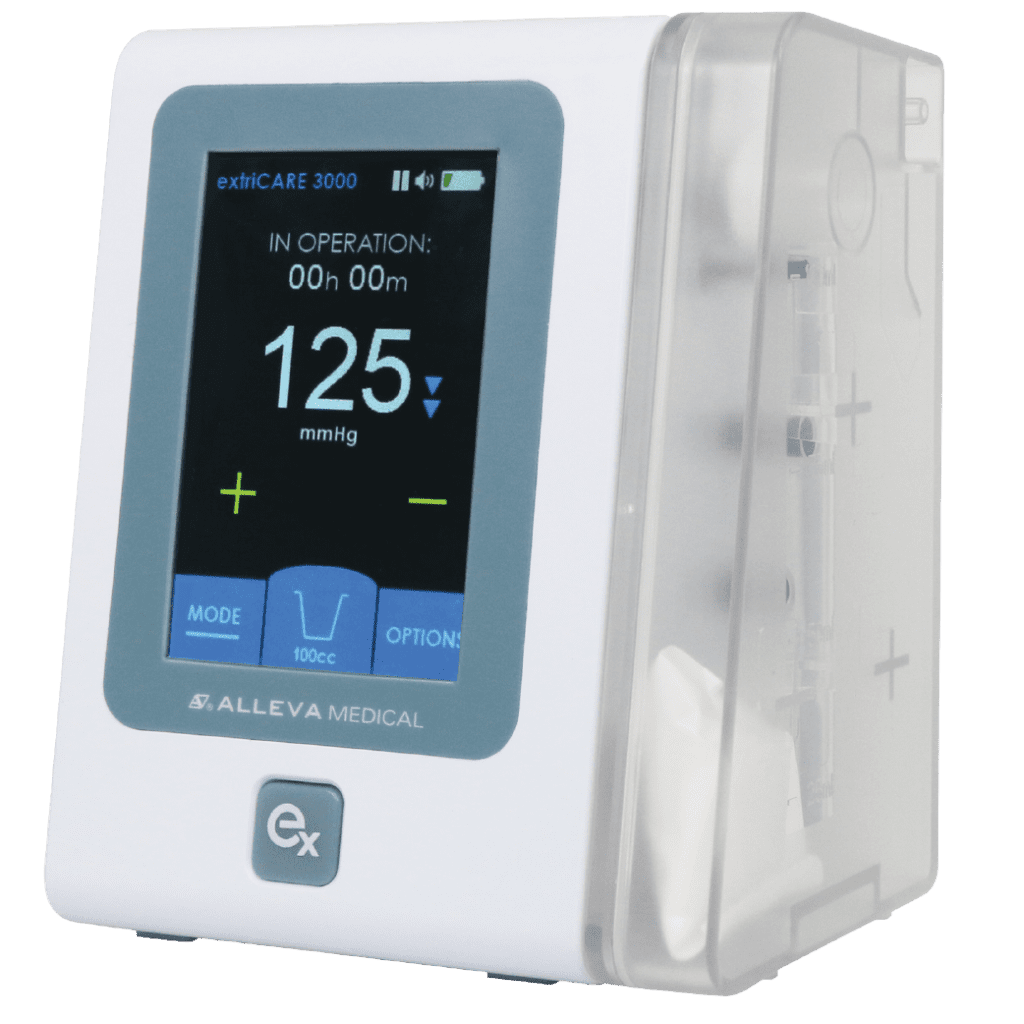
Read MoreExtriCARE USA Introduces the extriCARE 3000 Pump to NPWT Market
ExtriCARE USA, is pleased to announce that its innovative extriCARE 3000 pump has received FDA approval for use in the United States. The extriCARE 3000 pump is a state-of-the-art medical device designed to provide versatile and effective wound care treatment for patients. The extriCARE 3000 pump is a breakthrough in negative pressure wound therapy (NPWT)…
Read More