Archive for August 2019
Misonix Announces CE Mark Approval for Nexus Ultrasonic Platform
Misonix, Inc, a provider of minimally invasive therapeutic ultrasonic medical devices that enhance clinical outcomes, announced today that it has received Conformité Européene (CE) Mark approval for Nexus. The CE marking confirms that Nexus meets the requirements of the European Medical Devices Directive, which now allows Misonix to commercialize Nexus and its disposable products across the European…
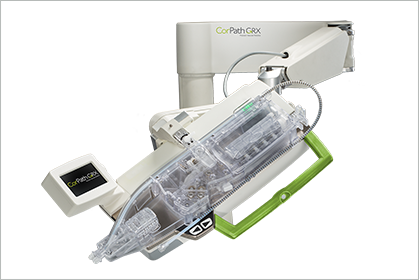
Read MoreCorindus Vascular Robotics to be Acquired by Siemens Healthineers for $1.1B
Corindus Vascular Robotics, a leading developer of precision vascular robotics, today announced that it has entered into a definitive merger agreement to be acquired by Siemens Healthineers AG. Under the terms of the merger agreement, Siemens Medical Solutions, a wholly-owned subsidiary of Siemens Healthineers AG, a German stock listed company, will acquire all issued and…
Read MoreNovel Imaging Method Limits Radiation Dosage from PET, SPECT Scans
A team of Japan-based researchers have created a medical camera capable of detecting and imaging radiotracers used in both PET and SPECT scans with limited radiation dosage. Detailed in Physics in Medicine & Biology, the Compton camera can detect gamma rays in both low and high energy ranges without the need of collimators. Its creators noted…
Read MoreEndotronix Announces FDA Approval to Begin Landmark PROACTIVE-HF Pivotal Trial for Cordella PA Pressure Sensor System
Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure, today announced it has received conditional Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin the multi-center PROACTIVE-HF trial of the Cordella™ Pulmonary Artery Pressure Sensor System (Cordella Sensor). The innovative trial…
Read More