Cardiovascular / Cardiology
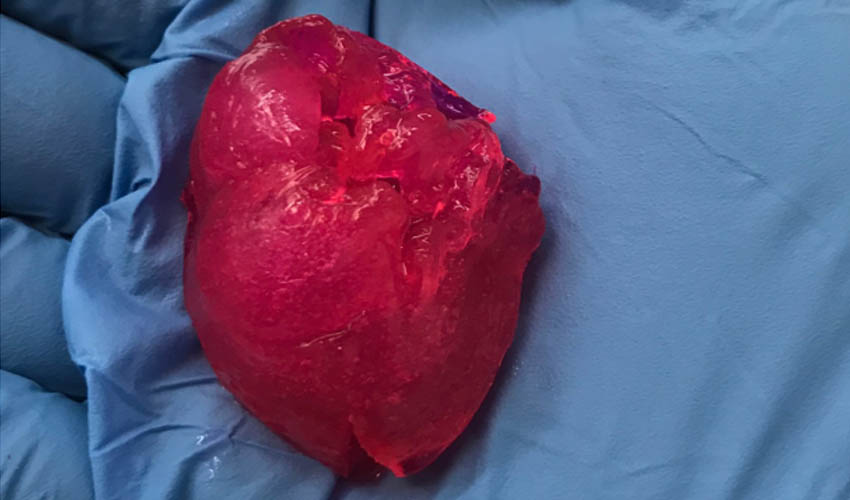
BIOLIFE4D Bioprints Small Human Heart for the First Time in the U.S.
BIOLIFE4D, one of the pioneers in the bioprinting field, has been able to bioprint a miniature human heart – making it the first U.S. company to successfully achieve this. The company’s mission is to create a fully functioning human heart through bioprinting and using patient’s own cells in order to eliminate the challenges of organ rejection…
Read MoreBiotronik First to Land MDR Certification for a High-Risk Device
Biotronik said that it is the first medtech manufacturer to receive European Medical Device Regulation (MDR) certification for a Class III (highest risk) medical device. The newly certified device is Biotronik’s Renamic programmer software, which enables physicians to program and test implanted cardiac devices such as pacemakers, implantable cardioverter-defibrillators and cardiac resynchronization therapy systems, according to…
Read MoreSaranas Launches Early Bird® Bleed Monitoring System in the U.S.
Saranas, Inc. announced the commercial launch of the Early Bird Bleed Monitoring System in the United States. The Early Bird is the first and only device for the monitoring and early detection of endovascular bleed complications through a novel application of bioimpedance sensors. Saranas will demonstrate the device in Booth 1755 at the Transcatheter Cardiovascular Therapeutics (TCT) Conference…
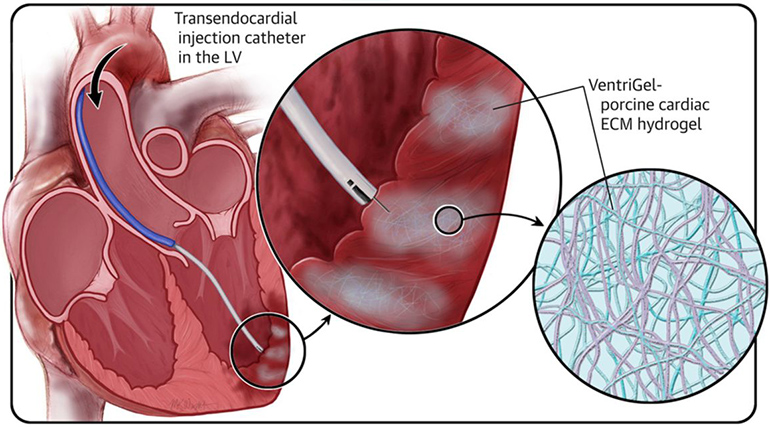
Read MoreFDA Phase 1 Trial Shows Hydrogel to Repair Heart Is Safe to Inject in Humans—A First
Ventrix, a University of California San Diego spin-off company, has successfully conducted a first-in-human, FDA-approved Phase 1 clinical trial of an injectable hydrogel that aims to repair damage and restore cardiac function in heart failure patients who previously suffered a heart attack. The trial is the first to test a hydrogel designed to repair cardiac…
Read MoreAbbott Wins CE Mark for Pediatric Heart Devices
Abbott said today that it won CE Mark approval for its Masters HP mechanical heart valve and Amplatzer Piccolo occluder. The Masters HP is the world’s smallest mechanical heart valve (15mm) and is designed for implantation in the mitral or aortic position to mimic a healthy heart valve by opening and closing to facilitate blood…
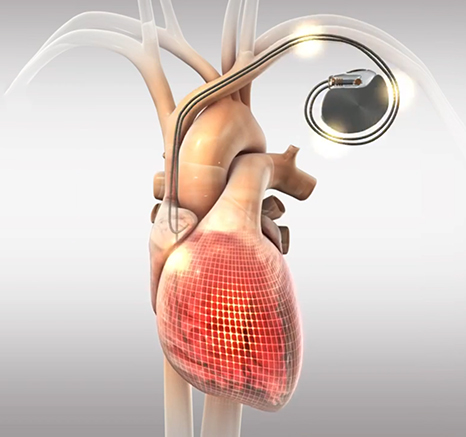
Read MoreFDA Grants EBR Systems Breakthrough Device Designation Status for the WiSE Cardiac Resynchronization Therapy (CRT) System
EBR Systems, Inc., developer of the world’s only wireless cardiac pacing system for heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the WiSE CRT System for the treatment of heart failure. The FDA created this designation and its associated program in 2017 for certain devices…
Read MoreTivus Ultrasound System for Pulmonary Arterial Hypertension Gets FDA Breakthrough Designation
SoniVie, an Israeli company developing a novel system for the treatment of PAH, today announced that it has been granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the Therapeutic Intra-Vascular Ultrasound (TIVUS) System in patients with PAH. “PAH is classified as a life-threatening or irreversibly debilitating disease because it is…
Read MorePacemaker-Like Implant Cleared in Europe to Lower Blood Pressure
Orchestra BioMed™, Inc., (“Orchestra BioMed” or the “Company”), a biomedical innovation company providing high-impact solutions for large unmet needs in procedure-based medicine, announced today that it has received CE Mark approval for its Moderato® implantable pulse generator system that delivers BackBeat Cardiac Neuromodulation Therapy™ (CNT) for treatment of hypertension while also providing standard pacemaker functions. Additionally,…
Read MoreFirst-ever: FDA Clears Biobeat’s Wearable Watch and Patch for Non-invasive Cuffless Blood Pressure Monitoring
Biobeat, a bio-medical technology company developing advanced sensing and remote monitoring solutions for patients, announced today that the U.S. Food and Drug Administration (FDA) has granted a 510K clearance for its patch and watch for measurement of blood pressure, oxygenation and heart rate in hospitals, clinics, long-term care and at home. Biobeat’s products enable cloud-based healthcare…
Read MoreMiracor’s Heart Attack Device Secures FDA Breakthrough Status
The concept of pressure-controlled intermittent coronary sinus occlusion, abbreviated to PICSO, has been around since the 1980s. PICSO entails placing a device in the coronary sinus to intermittently obstruct blood flow. Through the interference, PICSO may increase blood flow to parts of the heart affected by the myocardial infarction. Miracor, which received a CE mark for its…
Read More