Imaging Visualization & Navigation
HeartVista Receives FDA 510(k) Clearance for One Click™ Cardiac MRI Package, the First AI-assisted Cardiac MRI Scan Solution
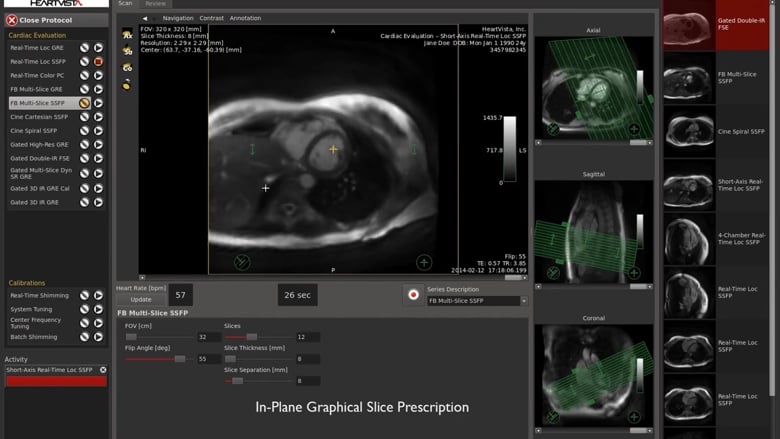
HeartVista, a pioneer in AI-assisted MRI solutions, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration to deliver its AI-assisted One Click™ MRI acquisition software for cardiac exams. Despite the many advantages of cardiac MRI, or cardiac magnetic resonance (CMR), its use has been largely limited due to a lack…
Read MoreLess Radiation and High Resolution – Canon Medical gains FDA Clearance for AI Image Reconstruction Technology
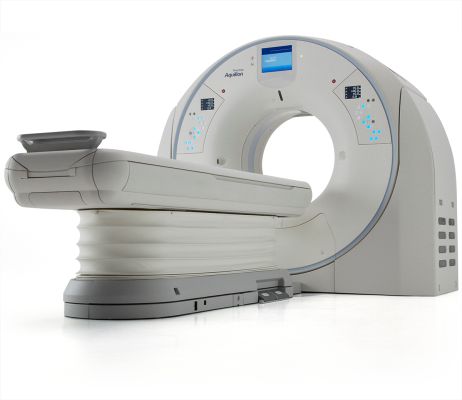
Canon Medical Systems USA, Inc. has received 510(k) clearance on its Advanced Intelligent Clear-IQ Engine (AiCE) for the Aquilion PrecisionTM further expanding access to its new deep convolutional neural network (DCNN) image reconstruction technology. This technology, now available on both the Aquilion Precision and Aquilion ONE / GENESIS EditionTM premium CT systems, uses a deep learning algorithm to differentiate…
Read MoreNIH Researchers Develop MRI with Lower Magnetic Field for Cardiac and Lung Imaging
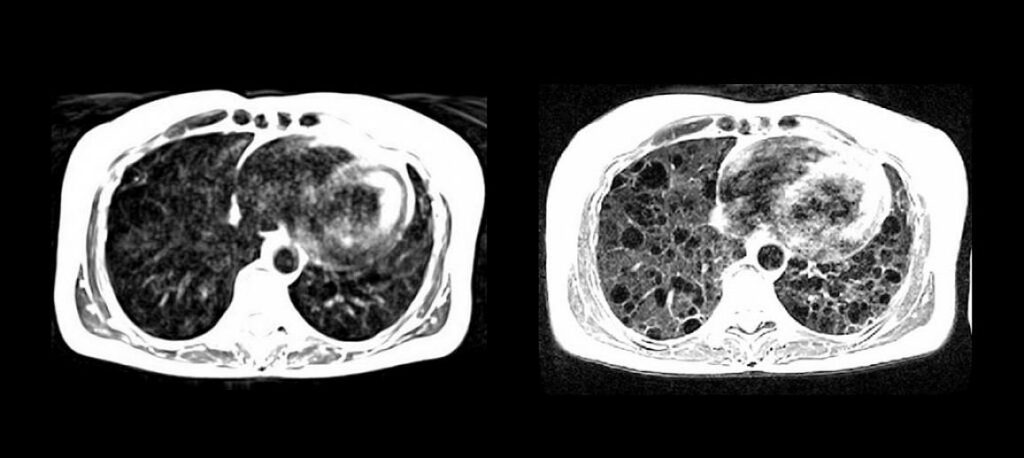
National Institutes of Health researchers, along with researchers at Siemens, have developed a high-performance, low magnetic-field MRI system that vastly improves image quality of the lungs and other internal structures of the human body. The new system is more compatible with interventional devices that could greatly enhance image-guided procedures that diagnose and treat disease, and…
Read MoreEpica Announces Multi-modality Mobile CT Imaging Platform has Gained FDA 510(k) Clearance
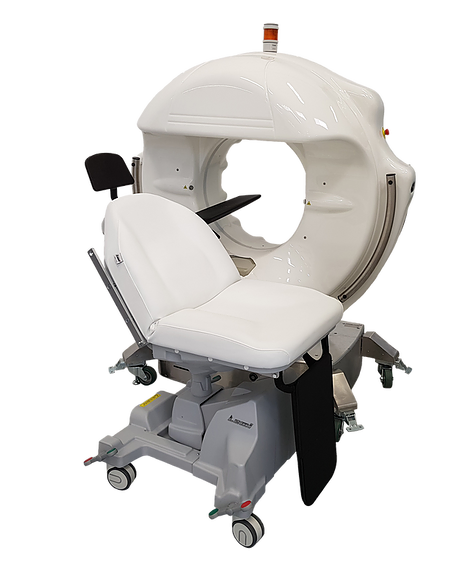
Epica International Inc. (Epica), received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its SeeFactorCT3™ Imaging Platform. SeeFactorCT3™ consists of 3 integrated imaging systems: CT, Fluoroscopy and Digital Radiography; a detachable patient table/chair, and sterile drape for interventional procedures. FDA clearance allows Epica to market these products in the United States.…
Read MoreNew Imaging Technique can Reveal Deep Tumors, and Help Attack Them
Tumors deep inside the body can be very difficult to spot, track, and study. The brain, being surrounded by a thick skull, is particularly challenging to image using light, so MRI and CT are currently the go-to imaging modalities when looking at deeply seated tissues. Now, researchers at Stanford University are reporting the development of…
Read MoreBrainlab Introduces Mobile Intraoperative Imaging Robot
Brainlab, the digital medical technology company, unveiled Loop-X™, the first mobile intraoperative imaging robot today at NASS 2019 in Chicago. Loop-X sits at the core of the Brainlab Digital Surgery portfolio for the rapidly evolving spinal market. Brainlab is leveraging its long history in 3D imaging and open integration to bring to market a new benchmark…
Read MoreSamsung to Unveil New Premium Ultrasound System at Society of Diagnostic Medical Sonography Conference of 2019
NeuroLogica, a subsidiary of Samsung Electronics Co. Ltd., announced that the Samsung Hera I10 ultrasound system has received FDA clearance and will be unveiled at the Society of Diagnostic Medical Sonography (SDMS) 2019 Annual Conference in National Harbor, MD. The Hera I10 is the latest addition to the Samsung Hera family of Premium Women’s Health ultrasound systems…
Read MoreFDA Clears ARTIS icono Family of Angiography Systems From Siemens Healthineers
The U.S. Food and Drug Administration (FDA) has cleared the ARTIS icono, a high-precision family of angiography systems from Siemens Healthineers that permit a wide range of minimally invasive procedures to be performed in a single interventional suite. The ARTIS icono biplane system is engineered for optimal utilization in neuroradiology and abdominal imaging, while the…
Read MoreGE Healthcare Receives FDA Clearance of First Artificial Intelligence Algorithms Embedded On-Device to Prioritize Critical Chest X-ray Review
GE Healthcare today announced the Food and Drug Administration’s 510(k) clearance of Critical Care Suite, an industry-first collection of artificial intelligence (AI) algorithms embedded on a mobile X-ray device. Built in collaboration with UC San Francisco (UCSF), using GE Healthcare’s Edison platform, the AI algorithms help to reduce the turn-around time it can take for…
Read MoreStryker announces definitive agreement to acquire Mobius Imaging & Cardan Robotics
Stryker (NYSE: SYK) announced today a definitive agreement to acquire Mobius Imaging, LLC, a leader in point-of-care imaging technology, and its sister company, GYS Tech, LLC (DBA Cardan Robotics), in an all cash transaction of approximately $370 million upfront and up to $130 million of contingent payments associated with development and commercial milestones. The acquisition…
Read More