Posts by rw-admin
Saluda Medical Receives FDA Approval for Evoke® System MRI Labeling
Saluda Medical, Inc. (“Saluda Medical”), a global medical device company revolutionizing the field of neuromodulation with an emerging portfolio of therapies driven by advanced closed-loop technologies, today announced that the U.S. Food and Drug Administration (FDA) has approved MRI conditional labeling for the Evoke® System, the first and only precision, dose-control spinal cord stimulation (SCS) therapy…
Read MoreChoiceSpine® Announces Standalone Indication for Blackhawk® Ti 3D Printed Cervical Spacer System
ChoiceSpine LLC, a privately held spinal device company held by Altus Capital Partners in Knoxville, TN, is pleased to announce today that it has received clearance from the U.S. Food and Drug Administration (FDA) to market the Blackhawk® Ti 3D Printed Cervical Spacer System with standalone clearance. Blackhawk® Ti utilizes preassembled integrated anchor technology with a proven…
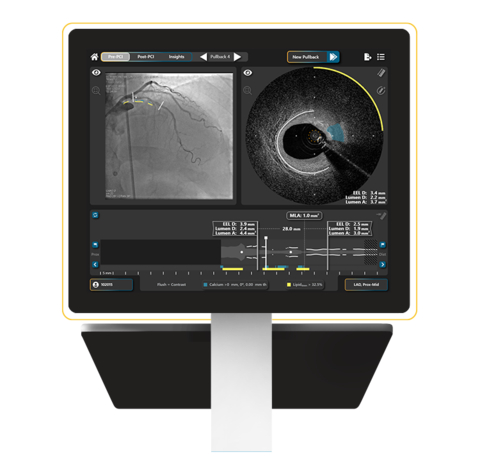
Read MoreSpectraWAVE Secures 510(k) Clearance to Add Saline Imaging and Expanded Artificial Intelligence Features to the HyperVue™ Imaging System
SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for product enhancements to the HyperVue™ Imaging System. The intravascular imaging system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of…
Read MoreCereVasc Receives FDA IDE Approval to Expand its Clinical Study of Patients with Normal Pressure Hydrocephalus Utilizing the Generation 2 eShunt® System
CereVasc, Inc., a clinical-stage, medical device company developing novel treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) supplement to permit the expansion of the study of the eShunt System in patients with Normal Pressure Hydrocephalus (NPH) to additional study participants and clinical sites.…
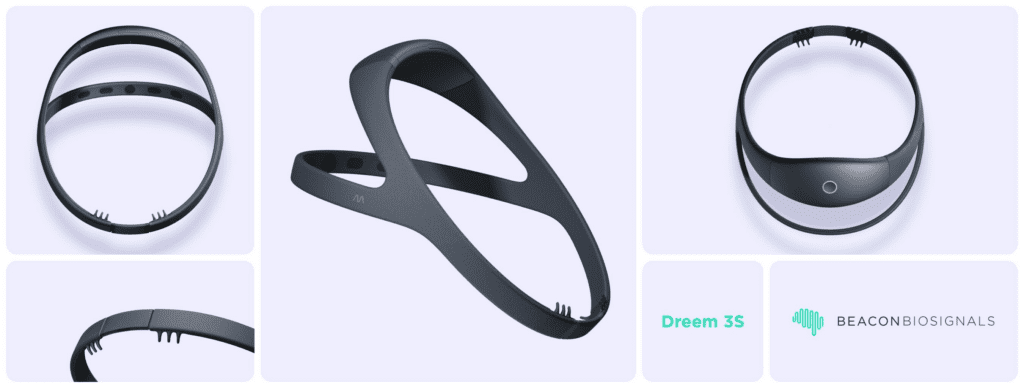
Read MoreBeacon Biosignals Receives FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S
Beacon Biosignals is thrilled to announce the milestone achievement of FDA 510(k) Clearance for the Dreem 3S, an advanced wearable headband with integrated machine learning algorithms to capture electroencephalogram (EEG) data from the brain to monitor sleep architecture and aid in the diagnosis of disturbed sleep. FDA Clearance marks it as equivalent to in-lab polysomnography for the…
Read MoreInspira™ Announces 510(k) FDA Submission of INSPIRA™ ART100 Towards Commercialization
Inspira™ Technologies OXY BHN Ltd. (Nasdaq: IINN, IINNW) (the “Company” or “Inspira Technologies”), a company aiming to bring a paradigm shift to acute respiratory care by empowering breathing without lungs, announced it had submitted its INSPIRA ART 100, a cardio-pulmonary bypass device, to the U.S. Food and Drug Administration (FDA) via the 510(k) pathway, with potential clearance…
Read MoreOrthofix Announces Leadership Changes
Orthofix Medical Inc. (NASDAQ: OFIX), a leading global spine and orthopedics company, today announced that Catherine Burzik, Chair of the Orthofix Board of Directors, has been appointed Interim Chief Executive Officer; Geoffrey Gillespie, Orthofix Vice President, Corporate Controller, has been appointed Interim Chief Financial Officer; and Puja Leekha, Orthofix Senior Vice President, Chief Ethics and…
Read MoreFDA Approves LimFlow System in Patients With Chronic Limb-Threatening Ischemia and No Suitable Endovascular or Surgical Revascularization Options
LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), announced today that the U.S. Food and Drug Administration (FDA) has approved the LimFlow System to help people with CLTI who have no other suitable endovascular or surgical treatment…
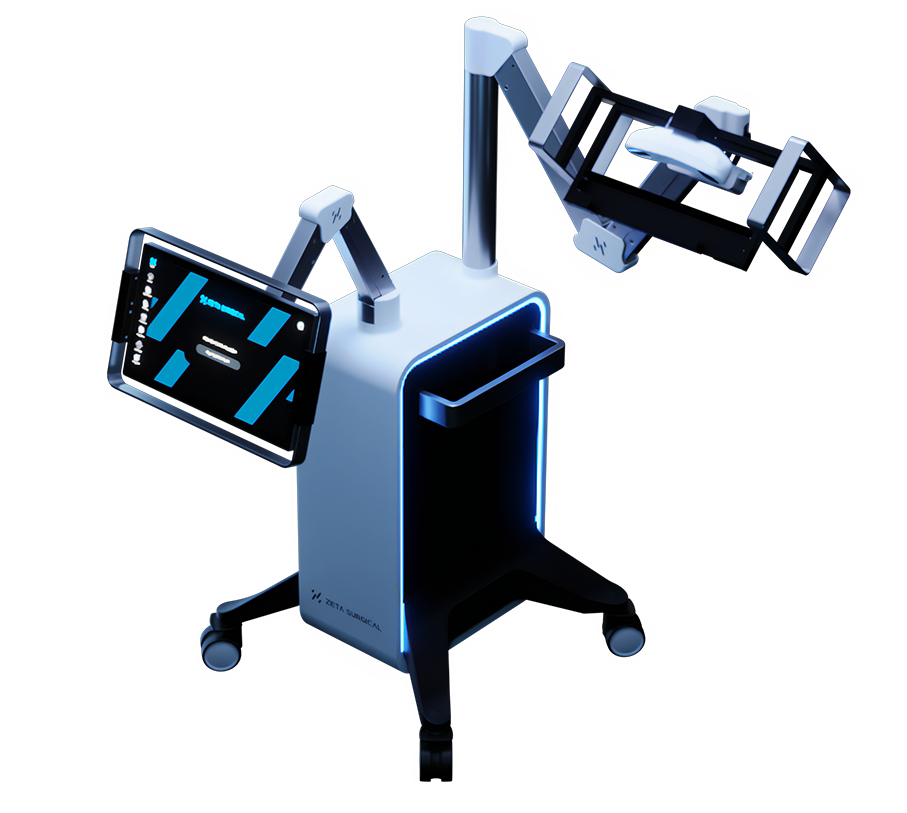
Read MoreZeta Surgical’s Mixed Reality Navigation System Receives FDA Clearance
Zeta Surgical, a surgical robotics and mixed reality company, announced today that the U.S. Food and Drug Administration has cleared the Zeta Cranial Navigation System, its mixed reality surgical navigation system. The Zeta Cranial Navigation System is a mixed-reality navigation system for neurosurgery that provides surgeons with “GPS-like” guidance with millimetric accuracy in real time. Zeta’s…
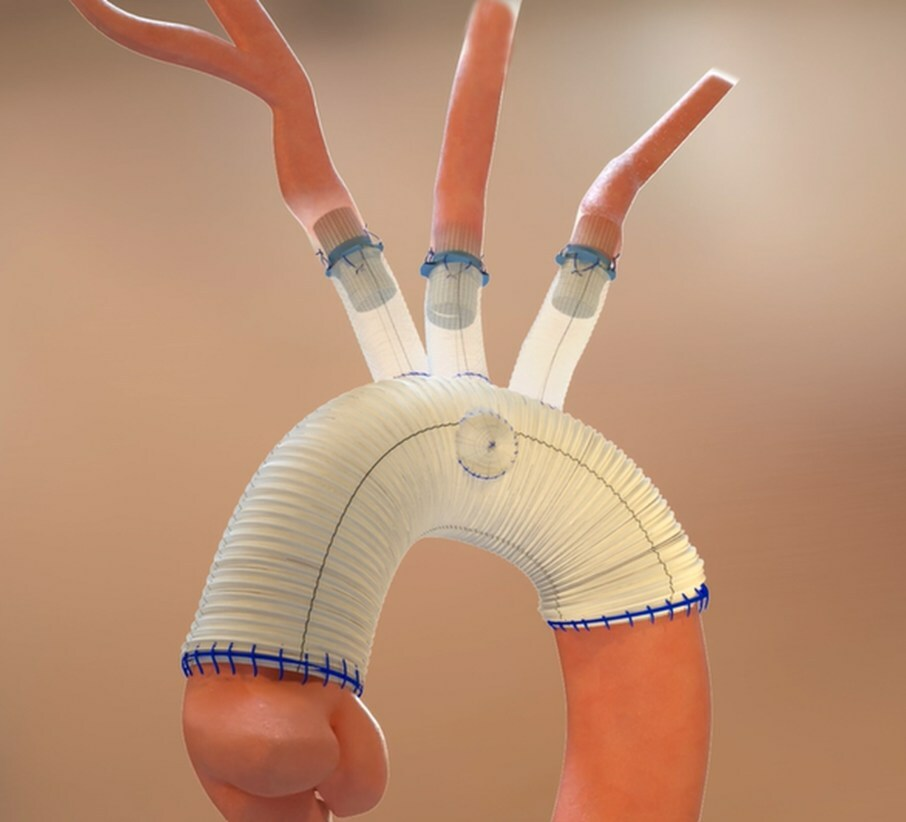
Read MoreAquedeon Medical, Inc. Receives FDA IDE Approval for the Duett Vascular Graft System
Aquedeon Medical, Inc., a Silicon Valley pioneering medical device company specializing in novel cardiothoracic solutions, is pleased to announce a significant milestone following receipt of FDA Investigational Device Exemption (IDE) approval to conduct a staged pivotal clinical trial for its Duett Vascular Graft System in the United States. The study will be initiated in the second…
Read More