Cardiovascular / Cardiology
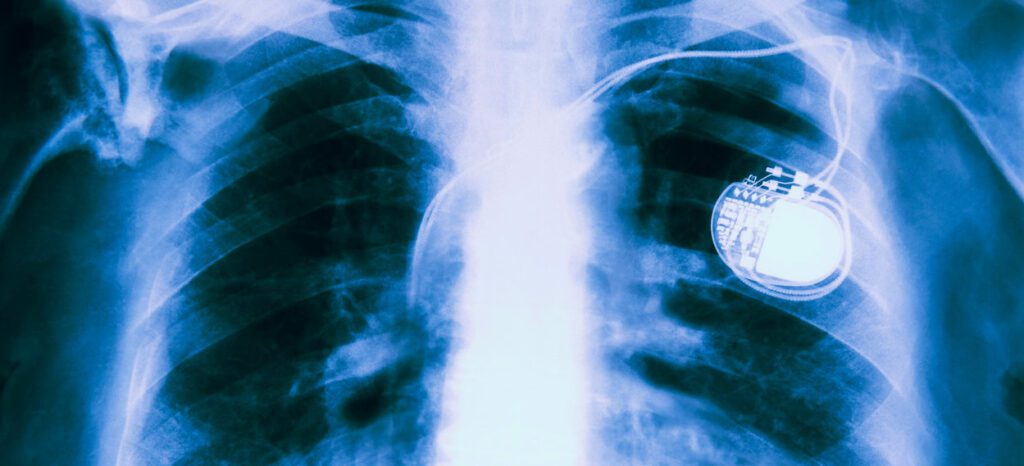
Warning from FDA: St. Jude Pacemaker Vulnerable to Hackers
When Vice President Dick Cheney asked his cardiologist to replace his WiFi-connected pacemaker with one without internet capability in 2007, assassination through medical device hacking seemed far-fetched. But this week, the U.S. Food and Drug Administration announced that almost half a million pacemakers made by Abbott are vulnerable to hackers. The FDA announced on Wednesday that patients with the Abbott (formerly St. Jude Medical) radio…
Read MoreHeartSciences Announces CE Mark and European Launch of MyoVista® High Sensitivity ECG Device
Westlake, Texas – August 17, 2017 – HeartSciences today announced the European launch of MyoVista® high sensitivity electrocardiograph (hsECG™) Testing Device, developed in response to the global unmet need for effective, low-cost, front-line screening of cardiac disease in both symptomatic and asymptomatic patients. MyoVista measures the heart’s energy during each heartbeat using a type of…
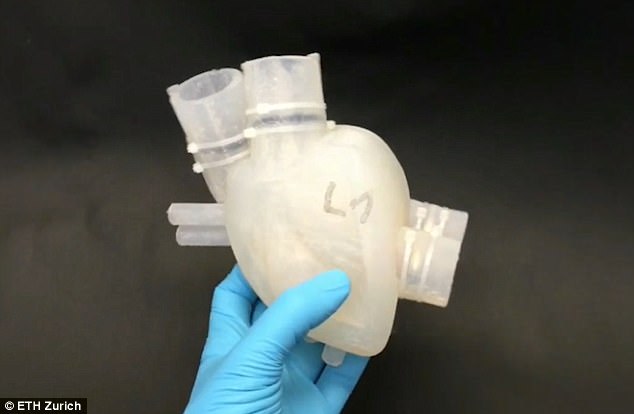
Read MoreScientists Create a 3D-Printed Silicone Artificial Heart
Heart transplants that do not require a human donor are a step closer after scientists 3D-printed a soft, artificial heart made of silicone. It closely imitates a human heart ‘in form and function’, according to its Swiss developers. Heart disease remains the number one killer worldwide, causing 17.3 million deaths each year – a problem exacerbated…
Read MorePhilips Picks Up Peripheral Thrombectomy Device Maker CardioProlific
Royal Philips (NYSE:PHG) last week said it paid an unspecified amount to acquire stealthy CardioProlific and the peripheral thrombectomy catheters it’s developing. Philips said the deal for Hayward, Calif.-based CardioProlific is complementary to its $2.16 billion acquisition of Spectranetics (NSDQ:SPNC) and its own line of image-guided therapies. “The acquisition of CardioProlific will further strengthen our innovation pipeline…
Read MoreSaranas Raises $4M to Advance Real-Time Detection Device of Internal Bleeding During Cardiac Procedures
HOUSTON–(BUSINESS WIRE)–Saranas, a medical device company with a new technology for real-time detection of internal bleeding during cardiac procedures, has closed a $4 million Series B round of financing. The funding will be used to advance the company’s Early Bird™ Bleed Detection device through final product testing and FDA submission later this year. Newly appointed…
Read MoreMan with Two Hearts Survives Heart Attack
At first there didn’t seem to be anything unusual about the man who, in 2010, reported to a Verona, Italy emergency room. He was short of breath, sweating, and had low blood pressure – cardiovascular trouble, no doubt. E.R. doctors see similar symptoms all the time. But this man was very different indeed. He had…
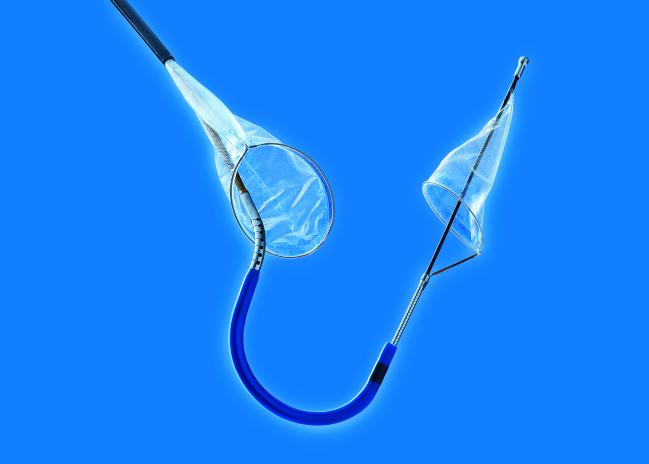
Read MoreFDA Clears Sentinel Cerebral Protection Device from Claret Medical
The US Food and Drug Administration (FDA) has cleared the Sentinel Cerebral Protection System (Claret Medical), a filter device used during transcatheter aortic valve replacement procedures to reduce the risk of stroke caused by embolic debris, the company announced today. The clearance comes after a mostly positive review by the agency’s advisory committee back in…
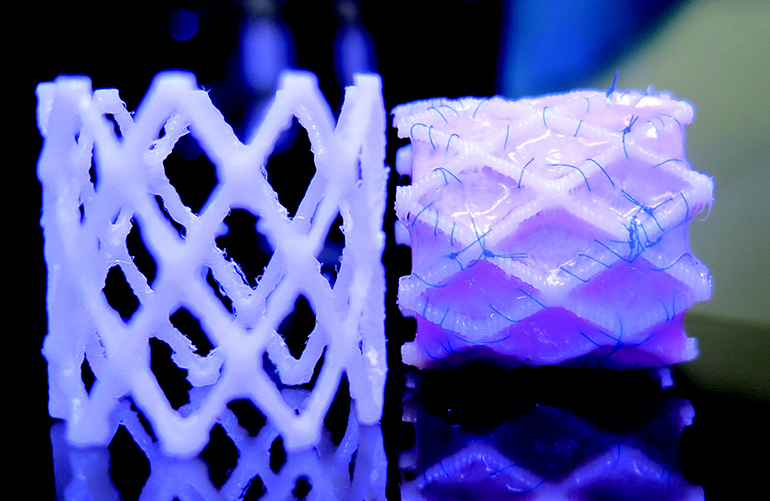
Read More3D Printed Stents for Children That Grow With Patient’s Vessels
Sadly, it is not only old people that receive cardiac stent implants. Often young children with certain cardiovascular conditions can benefit significantly from stent implantations and conduits, but because vasculature grows along with the rest of the body, the stents’ benefits can be short lived and not fully realized. Now researchers at Eindhoven University of Technology…
Read MoreAtaCor Medical has novel new method to pace the heart under development
We at Legacy MedSearch are sharing some wonderful technology news from AtaCor Medical written by: Rick Sanghera, Co-Founder & CEO at AtaCor Medical, Inc. Please be sure to click the link below to take you to the full article and a pretty amazing video. Make sure to share it with your connections! AtaCor Medical is developing a…
Read More26 Deaths Prompt Abbott Recall of Thoratec Heart Pumps
Abbott (NYSE:ABT) is recalling nearly 29,000 controllers for the HeartMate II implantable heart pump made by its Thoratec subsidiary after 26 patients died trying to change out the controllers on their own. The March 29 recall of 28,882 controllers for the HeartMate II left ventricular assist device follows 70 reports of “incidents in which the…
Read More