Cardiovascular / Cardiology
OpSens Announces FDA Clearance for the SavvyWire™ for Use in Transcatheter Aortic Valve Replacement (TAVR) Procedures
OpSens Inc., a medical device cardiology-focused company delivering innovative solutions based on its proprietary optical technology, announced that it has received 510(k) regulatory clearance from the U.S. Food & Drug Administration (“FDA”) for the SavvyWire™ (“SavvyWire”), its new guidewire for transcatheter aortic valve replacement procedures, or TAVR. “For OpSens, FDA clearance is a key milestone…
Read MorePotrero Medical Receives FDA Breakthrough Device Designation for Accuryn AKI Predict Algorithm
Potrero Medical announced that the FDA granted Breakthrough Device Designation for their AKI Predict machine learning algorithm, for the advanced prediction of acute kidney injury (AKI) associated with intra-abdominal hypertension (IAH) in cardiac post-surgical intensive care patients. Joe Urban, Potrero CEO stated “We celebrate the breakthrough designation, and we are excited about what this will…
Read MoreMiracor Medical Announces FDA IDE Approval For PiCSO® Pivotal Study
Miracor Medical SA (Miracor Medical) has announced the approval of an Investigational Device Exemption (IDE) from the FDA, enabling the company to initiate a pivotal study with its Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) technology. The PiCSO-AMI-II multicenter, randomized trial will enroll 300 patients with anterior ST-segment Elevation Myocardial Infarction (STEMI) presenting with TIMI flow…
Read MoreAncora Heart Receives Breakthrough Device Designation from FDA for the AccuCinch® Ventricular Restoration System
Ancora Heart, Inc., a company developing a novel device-based therapy to address heart failure, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the AccuCinch® Ventricular Restoration System. Currently being evaluated in the CORCINCH-HF pivotal clinical trial, the AccuCinch System is designed to provide a minimally invasive treatment option for…
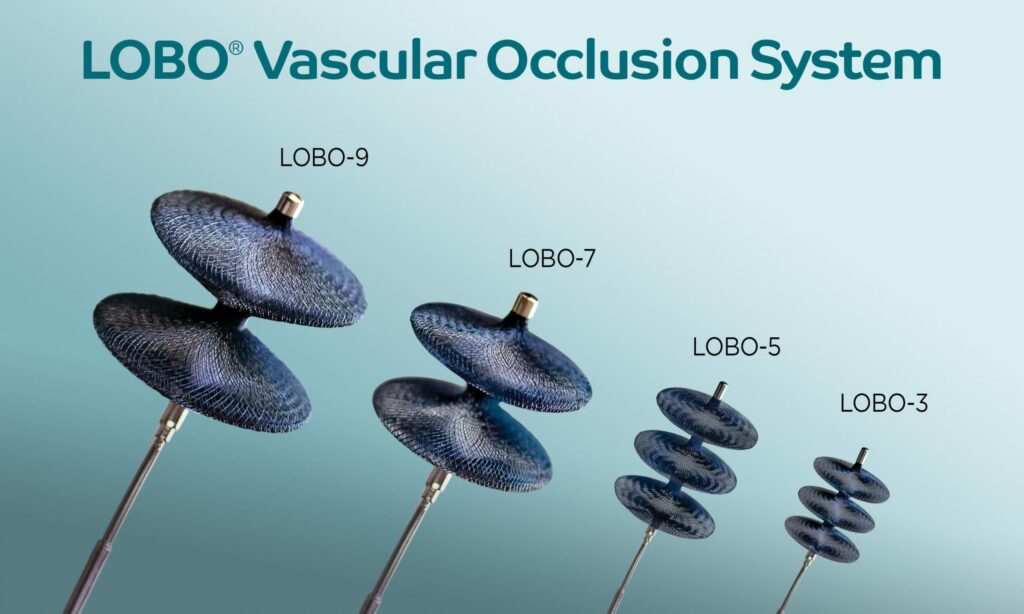
Read MoreOkami Medical Announces FDA 510(k) Clearance of the LOBO-7 and LOBO-9 Vascular Occluders to Address a Wide Range of Peripheral Embolization Cases
Okami Medical Inc., a medical device company focused on developing innovative solutions that address key unmet clinical needs in peripheral vascular intervention, announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the LOBO-7 and LOBO-9 Vascular Occluders, the latest addition to the company’s LOBO® Vascular Occlusion System. The LOBO (LOw-profile Braided Occluder) system, purpose-built for…
Read MoreCardio Flow, Inc., Announces FDA Clearance for FreedomFlow® Peripheral Guidewire, and their First Commercial Case Completed in U.S.
Cardio Flow, Inc., a medical device company and developer of minimally invasive peripheral vascular devices to treat peripheral artery disease (PAD), announced it recently received U.S. Food and Drug Administration (FDA) clearance for the company’s FreedomFlow® Peripheral Guidewire. The FreedomFlow guidewire is stainless steel core-to-tip design with a fixed distal-spring coil which was developed to…
Read MoreAcutus Medical Announces Agreements to Fund Strategic Growth Priorities
Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated, announced a commitment letter from Deerfield Management Company (“Deerfield”), to refinance its existing debt with a new longer-term credit facility, and in conjunction with the refinancing, a definitive agreement to sell the Company’s left-heart access portfolio to…
Read MoreArtio Medical Receives FDA Clearance for Solus Gold™ Embolization Device
Artio Medical, Inc., a medical device company developing innovative products for the peripheral vascular, neurovascular, and cardiology markets, announced it received US Food and Drug Administration (FDA) clearance for its Solus Gold Embolization Device, a next-generation product for peripheral vascular occlusion. “Current occlusion devices can be difficult to position in challenging anatomy, often require multiple implants,…
Read MoreTransMedics Receives FDA Clearance of OCS Lung Solution for Cold Preservation of Lungs
TransMedics Group, Inc. (“TransMedics”), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its OCS™ Lung Solution for use in transplants using cold storage techniques. The solution, which is also cleared as a component…
Read MoreCARMAT Announces the First Human Implant of its Total Artificial Heart in the United States
CARMAT, the designer and developer of the total artificial heart, aiming to fulfill an unmet medical need by providing a therapeutic alternative to people suffering from end-stage biventricular heart failure, announces the first implantation of its bioprosthetic artificial heart, Aeson, in the United States within the framework of the Early Feasibility Study (EFS). The implant…
Read More