Neuroscience
Rapid Medical Granted FDA Breakthrough Device Designation for Vasospasm Treatment
Rapid Medical’s Comaneci is the first device that allows physicians to monitor vessel expansion, apply incremental adjustments, and enhance treatment with combination therapeutics. With this unprecedented control, the FDA designated Comaneci as a breakthrough device–offering advantages over existing technology to improve safety and efficacy in cerebral vasospasm. Rapid Medical, a leading developer of advanced neurovascular…
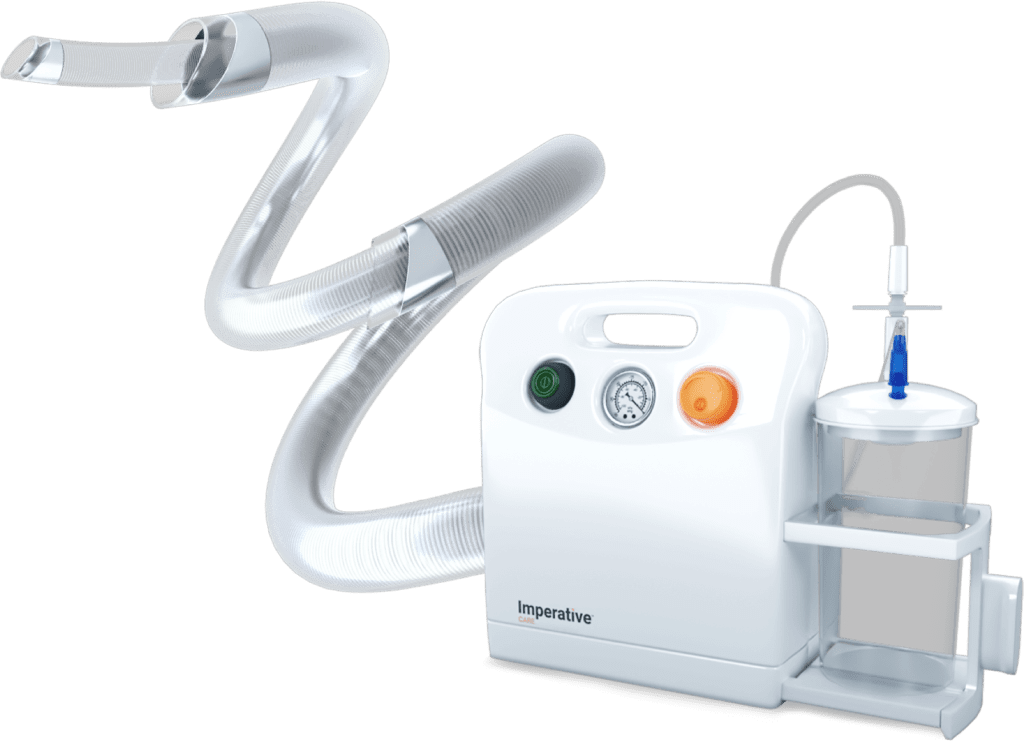
Read MoreImperative Care Launches Its Zoom POD™ for Stroke Treatment, the First and Only Filter to Capture Clot in the Sterile Field
Imperative Care, Inc. announced the launch of its Zoom POD™ Aspiration Tubing, the company’s latest innovation in elevating stroke care. The Zoom POD is the newest addition to Imperative Care’s Zoom Stroke Solution™, the company’s ischemic stroke product portfolio that includes the Zoom 88 Large Distal Platform for neurovascular access, four Zoom Aspiration Catheters in various…
Read MoreNeuros Medical Receives FDA Breakthrough Device Designation for its Novel Altius High Frequency Nerve Block System
Neuros Medical, Inc., announced today that it has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the use of its novel High-Frequency Nerve Block system as an aid in the management of chronic intractable pain of the lower limb of adult amputees. The FDA Breakthrough Device Program is intended to help patients…
Read MoreFDA Grants Breakthrough Device Designation for Anuncia’s Cerebral Spinal Fluid Treatment
Anuncia Inc., an emerging leader in Cerebral Spinal Fluid (CSF) management, received U.S. Food and Drug Administration (FDA) Breakthrough Device Designation for its ReFlow™ System Mini intended for the treatment of CSF disorders requiring shunting such as hydrocephalus, a debilitating and life-threatening condition affecting more than 1 million U.S. patients. Elsa Abruzzo, President of Anuncia Inc., stated,…
Read MoreNeuroOne Medical Announces First Human Commercial Use of its Evo Cortical Electrode at Mayo Clinic
NeuroOne Medical Technologies Corporation (OTCQB: NMTC; NeuroOne), a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, announced today the first human commercial use of its Evo Cortical Electrode at Mayo Clinic in Rochester, Minnesota. The procedure was performed by Jamie Van Gompel, M.D., from November 23rd through November 27th on a patient that had…
Read MoreMinnetronix Medical Wins FDA Clearance for Innovative Neurosurgical Access Platform
Minnetronix Medical, the company known for 25 years of developing and manufacturing products for medical device companies throughout the world, today announced that it has received FDA clearance for its first platform product: the MindsEye™ Expandable Port for neurosurgical procedures. This clearance represents an expansion of the company’s traditional offerings to include market-ready platforms. The…
Read MoreNevro Announces CE Mark Approval of Senza Omnia Spinal Cord Stimulation System to Treat Chronic Pain
Nevro Corp., a global medical device company that is providing innovative, evidence-based solutions for the treatment of chronic pain, today announced it has received CE mark approval for the Senza® Omnia™ Spinal Cord Stimulation (SCS) system. “Following a successful launch in the United States late last year, we are excited to now have approval for the Senza Omnia…
Read MoreGT Medical Technologies Announces FDA Clearance of Expanded Indication for GammaTile Therapy
GT Medical Technologies, Inc., a company dedicated to improving the lives of patients with brain tumors, today announced that the U.S. Food and Drug Administration (FDA) has cleared an expanded indication for GammaTile® Therapy. Patients with newly diagnosed malignant brain tumors are now eligible to receive the FDA-cleared surgically targeted radiation therapy (STaRT). “We are pleased…
Read MoreAptar’s Nasal Unidose Device Approved by U.S. FDA for First Nasal Rescue Treatment for Frequent Seizure Activity
AptarGroup, Inc., a global leader in drug delivery, consumer product dispensing and active packaging solutions, today announced that its patented Unidose Liquid System is the device delivering the first and only nasal rescue treatment approved by the U.S. FDA, which has recently launched in the U.S. to treat acute repetitive seizures in people living with…
Read MoreNeuronetics® and Success TMS Partner to Increase Patient Access to Leading Depression Treatment, NeuroStar® Advanced Therapy
Neuronetics, Inc., a commercial stage medical technology company focused on designing, developing and marketing products that improve the quality of life for patients who suffer from psychiatric disorders, today announced a partnership with Success TMS, a healthcare provider specializing in transcranial magnetic stimulation (TMS), a non-drug, non-invasive treatment for adult patients with Major Depressive Disorder…
Read More