Neuroscience
Quadriplegic Man Uses Exoskeleton to Walk and Move Arms Again for the First Time in Two Years
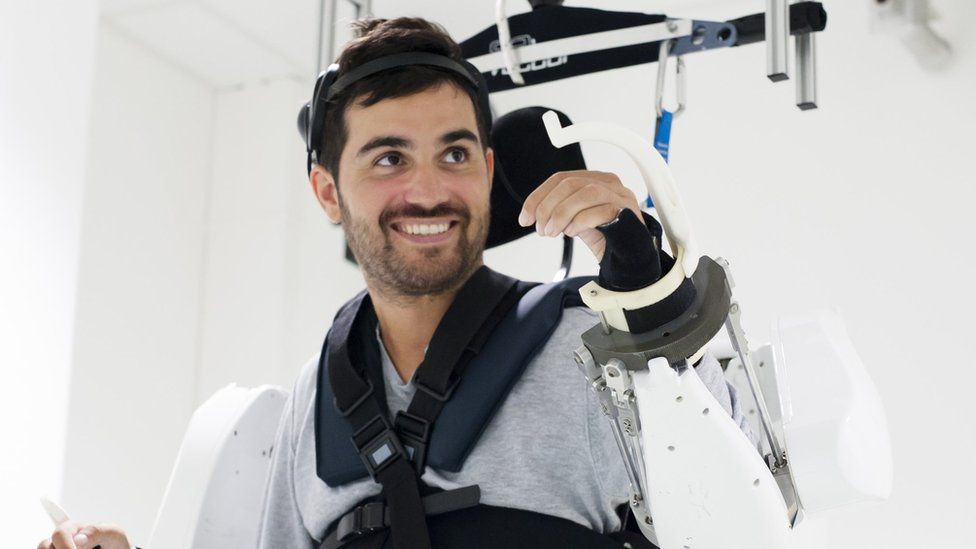
A four-limb robotic system controlled by brain signals helped a tetraplegic man to move his arms and walk using a ceiling-mounted harness for balance. While the early results are promising, the authors note that the system is a long way from clinical application and will require improvements before it becomes widely available. A whole-body exoskeleton,…
Read MoreAkili Interactive Develops ‘Prescription Video games’ for ADHD and Other Cognitive Disorders
Akili Interactive is developing a range of ‘prescription video games’ that combine specialised algorithms with cutting-edge gaming tech to improve cognitive function in various patient groups with neurological disorders. How exactly does this form of digital therapy work, and what is the potential for engaging game experiences to deliver cognitive benefits? When it comes to…
Read MoreFDA Grants Breakthrough Status to Aurora’s Concussion Treatment
There were 2.5 million emergency department visits related to traumatic brain injuries in the U.S. in 2014, according to the Centers for Disease Control and Prevention (CDC). Trends seen in the run up to 2014, the last year on which CDC has published data, suggest the figure may have risen since then. An earlier CDC report found mild traumatic…
Read MoreFirst Portable Brain-Computer Interface to Control Wheelchairs, TVs, Computers
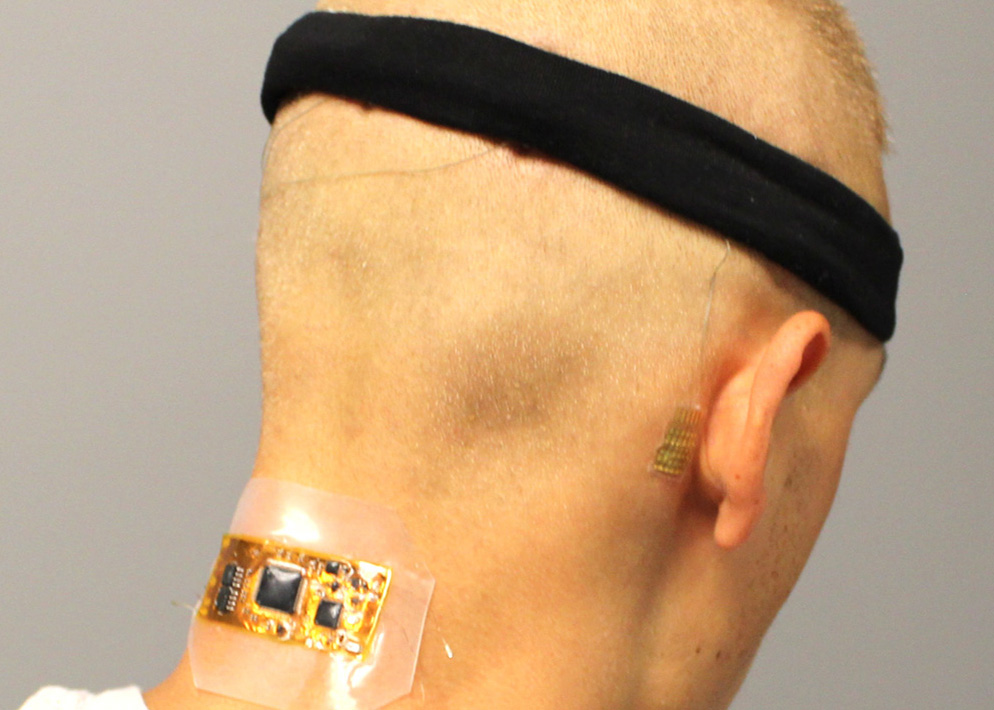
Brain-computer interfaces have the potential to give severely disabled people the ability to easily control their wheelchairs, televisions, and other devices. Existing technologies suffer from a number of limitations, though, making them impractical for real-world applications. One is that non-invasive brain wave monitoring currently requires large and uncomfortable electroencephalography caps with wet electrodes, wires, and…
Read MoreAxonics® Announces U.S. Food & Drug Administration Approval for its Sacral Neuromodulation System
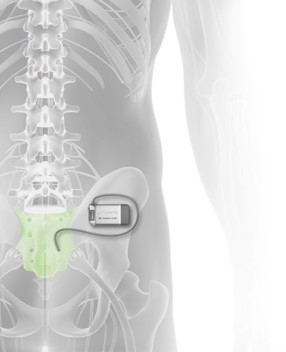
Axonics Modulation Technologies, Inc. (NASDAQ: AXNX), a medical technology company that has developed and is commercializing a novel implantable rechargeable sacral neuromodulation (“SNM”) device for the treatment of urinary and bowel dysfunction, today announced the approval of the Axonics r-SNM® System by the United States Food & Drug Administration (“FDA”). The Axonics System is the first rechargeable SNM system…
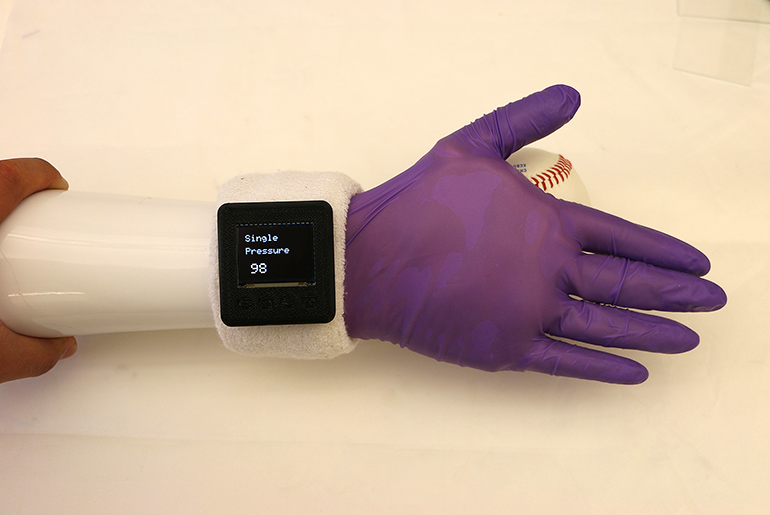
Read MoreMulti-Sensing Glove Makes Prosthetic Hands More Real
Engineers from Purdue University, University of Georgia, and University of Texas have combined forces to develop a glove that can be put over existing prosthetic hands to give them a more life-like feel and the ability to sense a variety of parameters. The glove is intended to improve a user’s ability to interact with others.…
Read MoreMedtronic to Distribute Viz.ai’s Stroke Imaging Software
Medtronic, a global leader in medical technology, and Viz.ai, the emerging leader in applied artificial intelligence (AI) in stroke care, have partnered to accelerate the adoption of Viz.ai’s new technology, which helps synchronize stroke care and decrease time to treatment, potentially improving outcomes for patients. Viz.ai’s technology uses artificial intelligence to identify suspected large vessel…
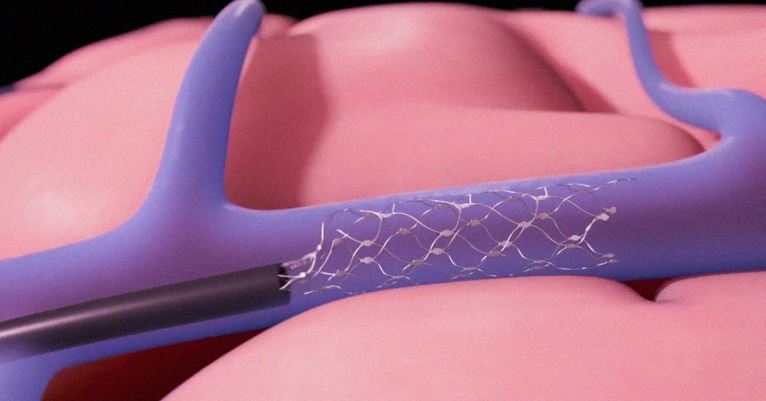
Read MoreFirst-Ever Clinical Trial Initiated for Neural Interface that Turns Thoughts into Speech
Synchron, Inc. today announced the initiation of the first clinical trial for the Stentrode™, a minimally-invasive neural interface technology being investigated for restoration of communication in people with severe paralysis. The trial will evaluate the safety of Thought-to-Text™ technology in patients, by assessing the Stentrode implant in combination with BrainOS™ software. The Stentrode is designed to record brain activity…
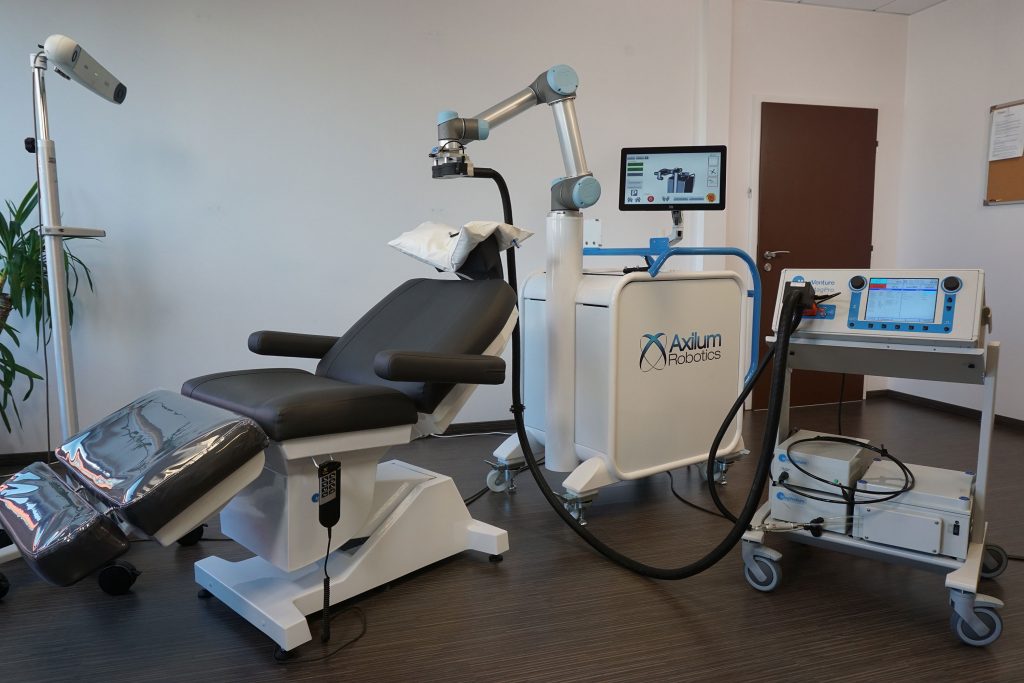
Read MoreAxilum Wins 510(k) Clearance for Robotic TMS System
Axilum Robotics, which specializes in the development of medical robots, announced that two weeks after the CE mark, it has received 510(k) clearance from the U.S. Food and Drug Administration to market the TMS-Cobot TS MV for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system. The company successfully developed…
Read MoreIRRAS’s Intracranial Bleeding Management Device Used on First U.S. Patient
IRRAS AB, a commercial-stage medical-technology company, today announced that the first patient in the United States was successfully treated with IRRAflow, the company’s initial product that offers an innovative, therapeutic approach to the fluid management of patients with intracranial bleeding. “The US is the largest market for intracranial procedures, and we are making significant progress engaging neurocritical care centers and specialty hospitals across the country,” said Kleanthis G. Xanthopoulos, Ph.D.,…
Read More