Neuroscience
WISE Receives FDA Clearance for its WISE Cortical Strip Bringing Neuromonitoring to the Next Level
WISE Srl, a medical device company developing next-generation implantable electrodes for neuromonitoring, neuromodulation and brain-machine interfacing (BMI), announced the FDA clearance of its WISE Cortical Strip (WCS®), a single use medical device intended to be used on the brain surface for Intra Operative Neurophysiological Monitoring (IONM). The WISE Cortical Strip is the first product receiving FDA…
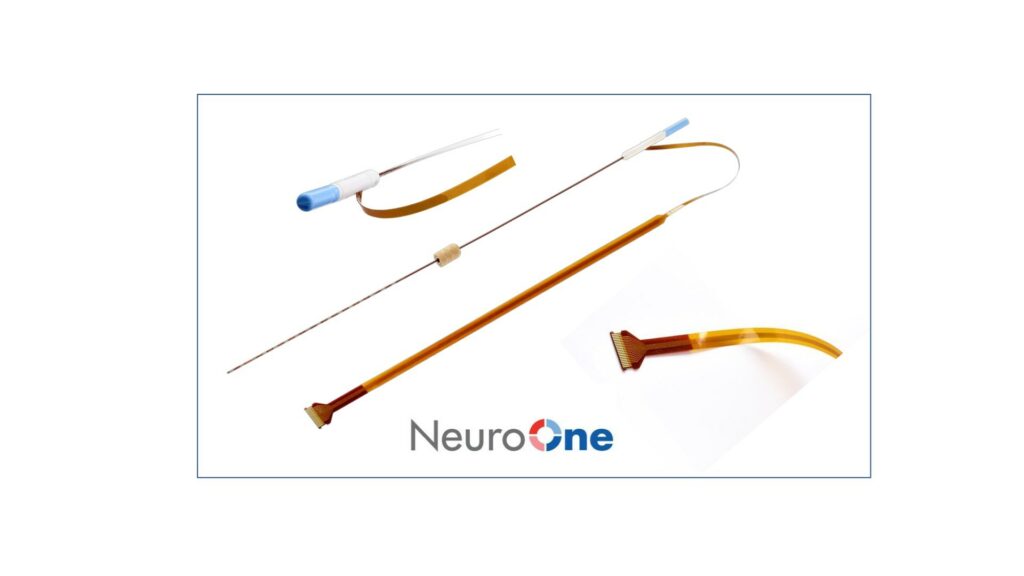
Read MoreNeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
NeuroOne Medical Technologies Corporation, a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Evo sEEG Electrode technology for temporary (less than 30 days) use with recording, monitoring, and stimulation equipment…
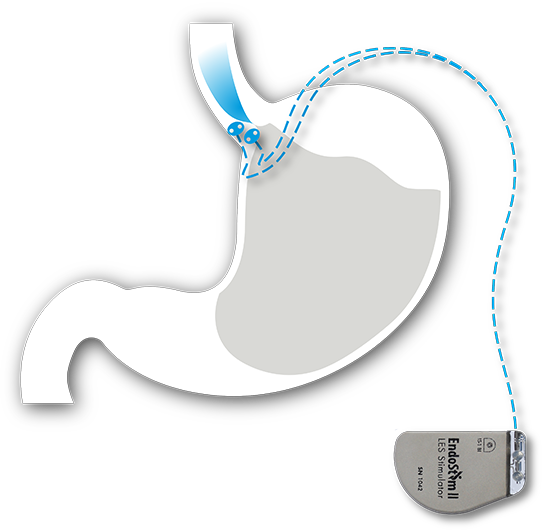
Read MoreEndoStim Receives FDA Breakthrough Device Designation for the EndoStim System for the Treatment of Drug Refractory GERD
EndoStim, a medical device company developing and commercializing a first-in-class implantable neurostimulation treatment for drug refractory gastroesophageal reflux disease (GERD), announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the Company’s EndoStim System. FDA Breakthrough Device Designation is intended to provide patients and health care providers with timely access to…
Read MoreAxoft Launches Brain Implant Technology to Treat Long-Term Neurological Disorders and is Granted FDA Breakthrough Device Designation
Axoft, a neurotechnology company, launched and announced FDA Breakthrough Device designation for its brain-machine interface (BMI) to better treat neurological disorders. The company secured $8 million in capital to fund pre-clinical studies with the FDA and to scale up prototypes of its neural implants “as soft as the brain.” The seed round investment, led by…
Read MoreNevro Announces FDA Approval of HFX iQ™ Spinal Cord Stimulation System to Personalize the Treatment of Chronic Pain
Nevro Corp. (NYSE: NVRO), a global medical device company that is delivering comprehensive, life-changing solutions for the treatment of chronic pain, announced that it has received approval from the U.S. Food and Drug Administration (FDA) for the Senza HFX iQ spinal cord stimulation (SCS) system. Senza HFX iQ is the first and only Artificial Intelligence-based SCS system that…
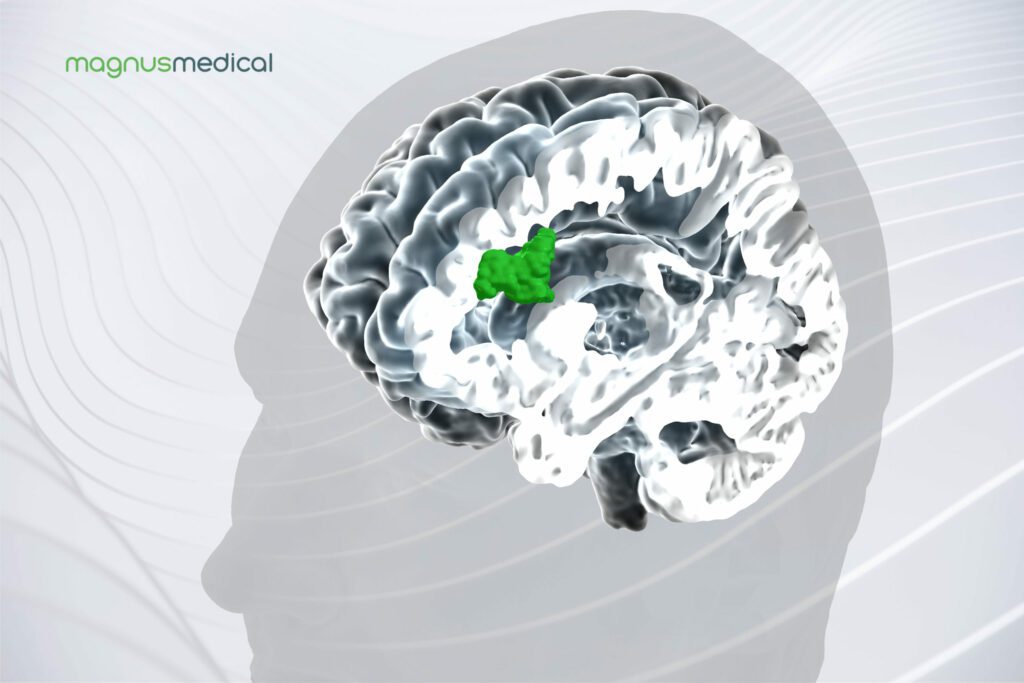
Read MoreMagnus Medical Receives FDA Clearance for the SAINT Neuromodulation System
Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for treatment of neuropsychiatric disorders, announced it received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the SAINTTM Neuromodulation System for the treatment of major depressive disorder (MDD) in adults who have failed to achieve satisfactory improvement from prior antidepressant…
Read MoreCereVasc Announces FDA Approval of Second IDE Study of the eShunt® System
CereVasc, Inc., a privately held, clinical-stage, medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application to initiate a pilot trial of the eShunt® System in patients who develop communicating hydrocephalus post aneurysmal subarachnoid hemorrhage (paSAH). “We are…
Read MoreClearPoint Neuro Announces FDA Clearance for ClearPoint Maestro™ Brain Model
ClearPoint Neuro, Inc., a global therapy-enabling platform company providing navigation and delivery to the brain, announced it has received 510(k) clearance for its ClearPoint Maestro™ Brain00 Model. Maestro is intended for automatic labeling, visualization, volumetric and shape quantification of segmentable brain structures from a set of MRI images. This software is intended to automate the…
Read MoreCognito Therapeutics Announces Proprietary Gamma Sensory Stimulation for 6-Months Reduces White Matter Atrophy in Alzheimer’s Disease Patients
Cognito Therapeutics, announced that its proprietary gamma sensory stimulation at 40Hz over a 6-month period reduced white matter atrophy in the brain for patients with Alzheimer’s Disease, according to new data presented at the Alzheimer’s Association International Conference 2022. Alzheimer’s Disease (AD) is the most common form of dementia. Although white matter atrophy is observed…
Read MoreBlackrock Neurotech Acquires Spatial Computing Software Firm MindX to Commercialize Full-Stack Brain-Computer Interface Product
Blackrock Neurotech, a leading implantable brain-computer interface (“BCI”) technology backed by world-renowned investors Peter Thiel and Christian Angermeyer, announced the acquisition of spatial computing software firm MindX, integrating the Company’s innovative augmented reality (“AR”) and artificial intelligence (“AI”) technology with Blackrock’s BCI hardware. MindX was created in 2017 by serial health-tech entrepreneur Julia Brown and Catalio Capital Management, a multi-strategy…
Read More