Surgery and Surgical Robotics
Medtronic Warns of Mazor X Detachment Issue in Robotic Surgery
Users of Medtronic’s Mazor X Surgical System may experience a hardware detachment problem in which a system piece unexpectedly releases from the OR table, according to an urgent field safety notice issued by the company in December. Medtronic said it had received seven complaints of the issue occurring as of Nov. 13, none of which involved patient…
Read MoreInterscope Announces New FDA Clearance of the EndoRotor, for Use in Airway Procedures Including in Interventional Pulmonology
Interscope, Inc. announced today the receipt of marketing clearance from the FDA for the EndoRotor® System to commercialize Pulmonary indications. Interscope innovated the first flexible microdebrider for use by medical specialists in the digestive tract with reported results reducing the need for surgery in recurrent adenoma and removal of walled off necrosis. The company now looks…
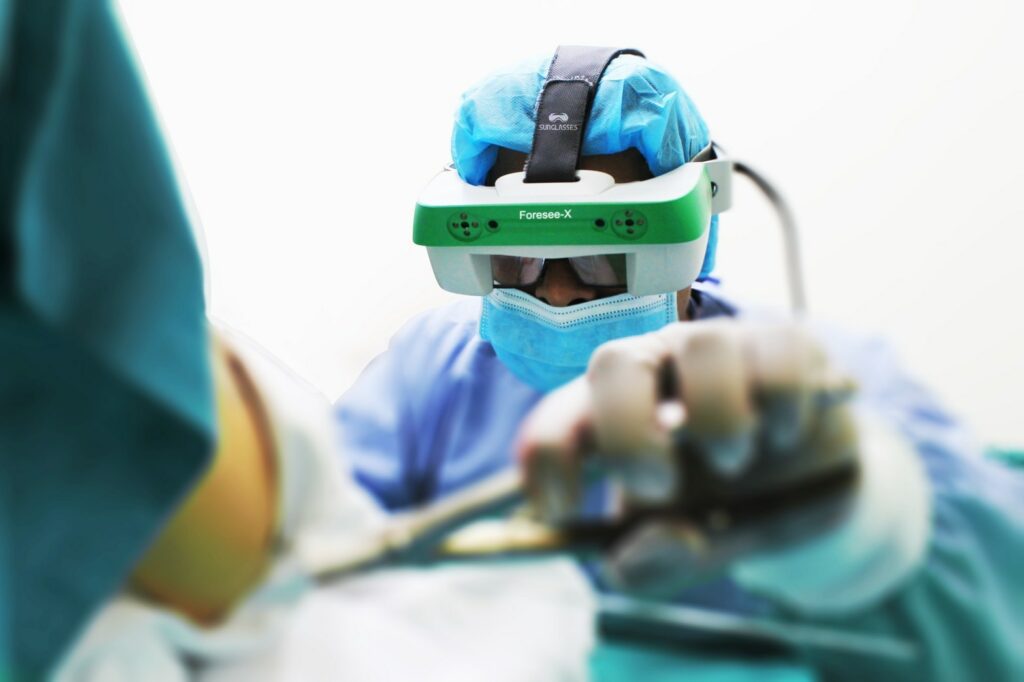
Read MoreForesee-X Augment Reality Solution from SURGLASSES Is Registered with the FDA and Ready for Trauma Treatment
Foresee-X is a set of smart surgical glasses with functionality based on augmented and mixed reality technologies. This device was developed by SURGLASSES, also known as Taiwan Main Orthopaedic Biotechnology Co. Ltd., and has received IEC60601-1-2 and ISO 13485 certifications. It is designed to bring a higher level of support to surgical procedures for trauma cases.…
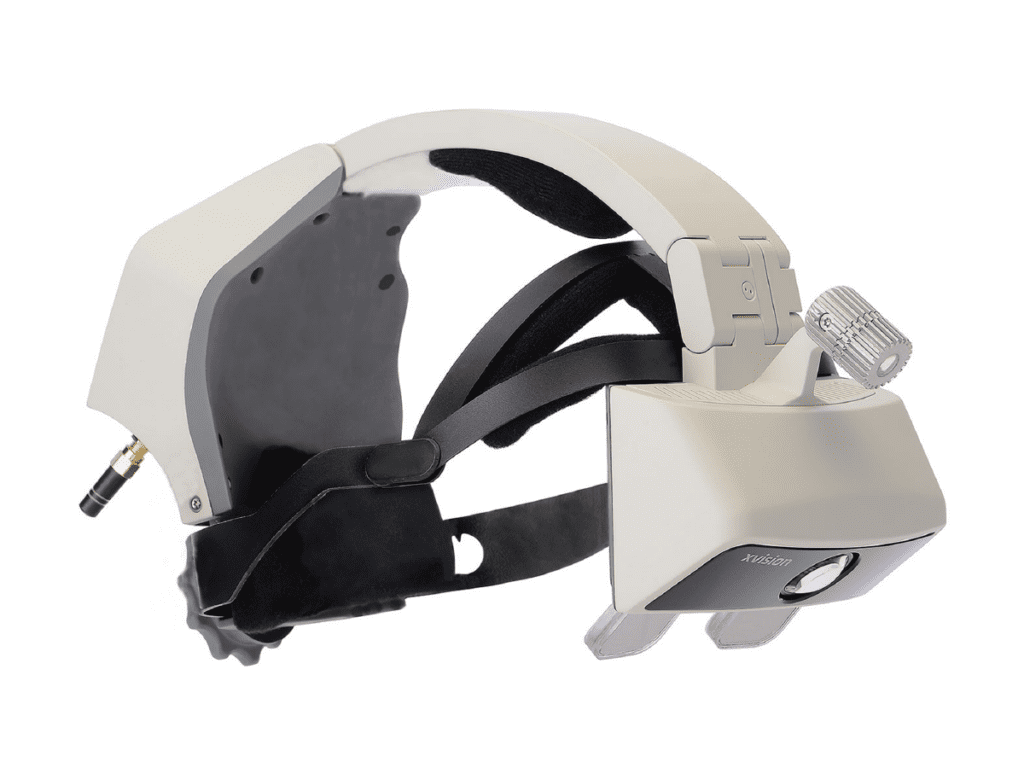
Read MoreAugmedics Announces FDA 510K Clearance and U.S. Launch of xvision, the First Augmented Reality Guidance System for Surgery
Augmedics, a pioneer in augmented reality surgical image guidance, has announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the U.S. launch of its groundbreaking xvision Spine system (XVS), the first AR guidance system to be used in surgery. xvision Spine allows surgeons to visualize the 3D spinal anatomy of a patient during surgery…
Read MoreIRRAS Receives Renewed CE Mark for the IRRAflow Catheter
IRRAS, a global healthcare company with a comprehensive portfolio of innovative products for neurocritical care, announced today that it received CE Mark approval for its IRRAflow®catheter. This CE Mark complements the two CE Marks previously obtained for the IRRAflow system’s tube set with digital pump and control unit, and allows IRRAS to once again commercially market the IRRAflow system in…
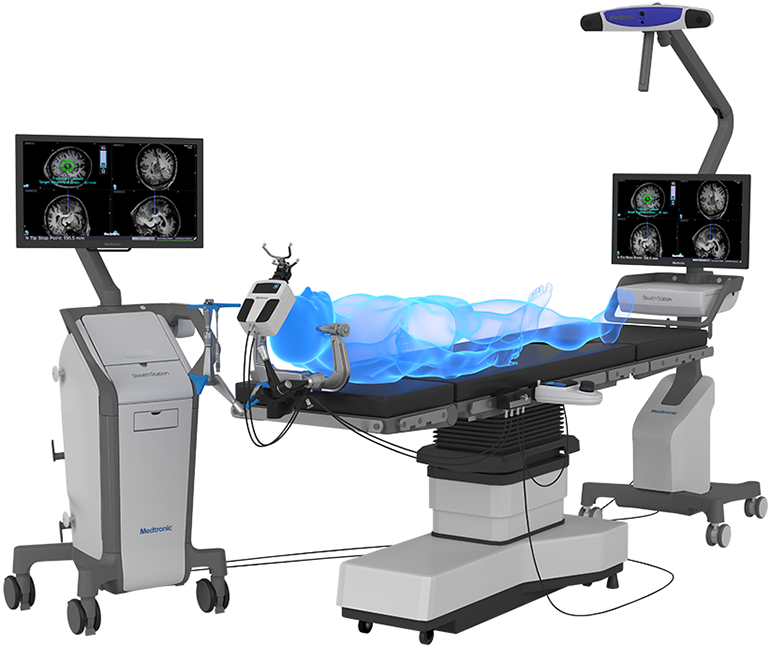
Read MoreMedtronic Expands Surgical Synergy(SM) with FDA Clearance of the Stealth Autoguide System for Cranial Procedures
Medtronic plc announced that the U.S. Food and Drug Administration (FDA) recently cleared the Stealth Autoguide™ system, the first cranial robotic platform that integrates with Medtronic’s enabling technology portfolio to create an end-to-end procedural solution. The Stealth Autoguide Platform is a robotic guidance system intended for the spatial positioning and orientation of instrument holders or…
Read MoreAptar’s Nasal Unidose Device Approved by U.S. FDA for First Nasal Rescue Treatment for Frequent Seizure Activity
AptarGroup, Inc., a global leader in drug delivery, consumer product dispensing and active packaging solutions, today announced that its patented Unidose Liquid System is the device delivering the first and only nasal rescue treatment approved by the U.S. FDA, which has recently launched in the U.S. to treat acute repetitive seizures in people living with…
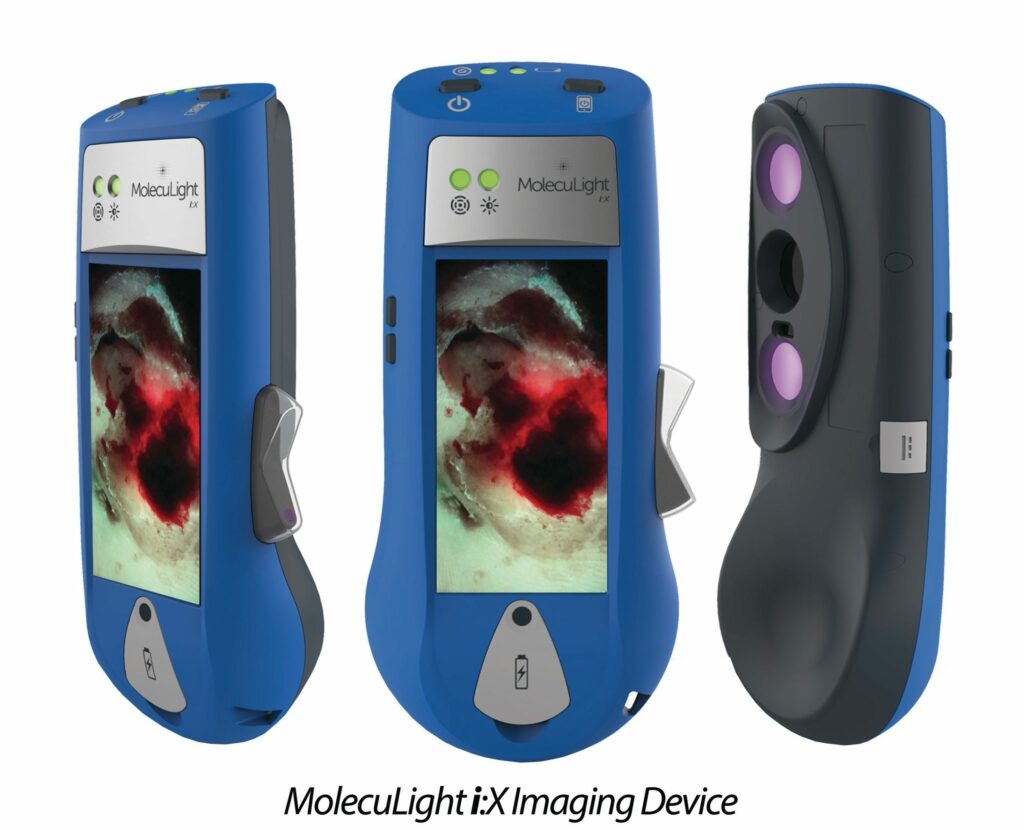
Read MoreMolecuLight Receives FDA 510(k) Clearance for its i:X Handheld Fluorescence Imaging Device for Wound Management
MolecuLight Inc., the world’s leader in handheld fluorescence imaging for real-time visualization of fluorescence in wounds, has received FDA 510(k) clearance for its i:X® handheld fluorescence imaging device for use in the detection of wounds containing bacteria. This FDA 510(k) clearance is an expansion of the original de novo clearance for the MolecuLight i:X platform, granted on August 14, 2018. The…
Read MoreVicarious Surgical Wins FDA Breakthrough Designation for Surgical Robot
Vicarious Surgical Inc., the leading innovator in virtual reality and robotics for minimally invasive surgical treatments, announced today they were recently granted the Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA). The FDA Breakthrough Devices Program is intended to help patients receive more timely access to breakthrough technologies that have the potential…
Read MoreFDA Has Cleared Two Intuitive Technologies for Use in Sealing Procedures
Intuitive, a global technology leader in minimally invasive care and the pioneer of robotic-assisted surgery, announced U.S. Food and Drug Administration clearance of two innovative technologies for two of the company’s da Vinci® surgical systems to help improve procedures that require sealing. Intuitive’s E-100 generator is its first internally developed robotic generator to power two key…
Read More