Surgery and Surgical Robotics
Medtronic to Distribute Viz.ai’s Stroke Imaging Software
Medtronic, a global leader in medical technology, and Viz.ai, the emerging leader in applied artificial intelligence (AI) in stroke care, have partnered to accelerate the adoption of Viz.ai’s new technology, which helps synchronize stroke care and decrease time to treatment, potentially improving outcomes for patients. Viz.ai’s technology uses artificial intelligence to identify suspected large vessel…
Read MoreMedtronic Partnership Reveals New Details About Surgical Robotics Project
Medtronic won’t unveil its new robot-assisted surgery platform until the fall, but a recent announcement from the company offered a sneak peek at what’s coming. The medical device giant yesterday disclosed a partnership with Karl Storz SE & Co. (Tuttlingen, Germany) to use Karl Storz’s three-dimensional vision systems and visualization components into Medtronic’s upcoming robot-assisted surgical…
Read MoreNexus Ultrasonic Surgical Platform from Misonix FDA Cleared
Misonix, Inc., a provider of minimally invasive therapeutic ultrasonic medical devices that enhance clinical outcomes, today announced that it received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for Nexus, its revolutionary ultrasonic surgical platform. Misonix will commence the commercialization of the Nexus platform in the United States in July. Nexus is a…
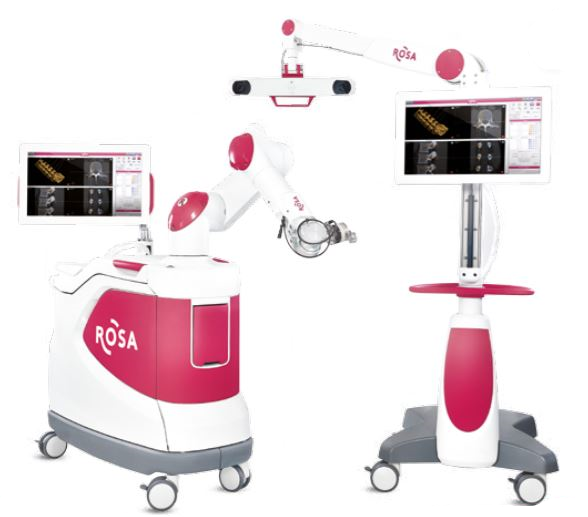
Read MoreZimmer Biomet Receives FDA Clearance for Robotic ROSA One Spine System
Zimmer Biomet Holdings, Inc., a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® ONE Spine System for robotically assisted minimally invasive and complex spine surgeries, strengthening the Company’s comprehensive ROSA® ONE Brain and ROSA® Knee portfolio. ROSA ONE Spine combines robotics and navigation while delivering a unique real-time patient…
Read MoreFDA Warns Against Using Surgical Robots in Cancer Surgeries
The Food and Drug Administration on Thursday warned against the use of robotically assisted devices for mastectomies and other cancer surgeries, asserting the products may pose safety risks and result in poor outcomes for patients. The agency said it decided to issue its warning after reviewing studies suggesting that robotically assisted devices were being used to perform…
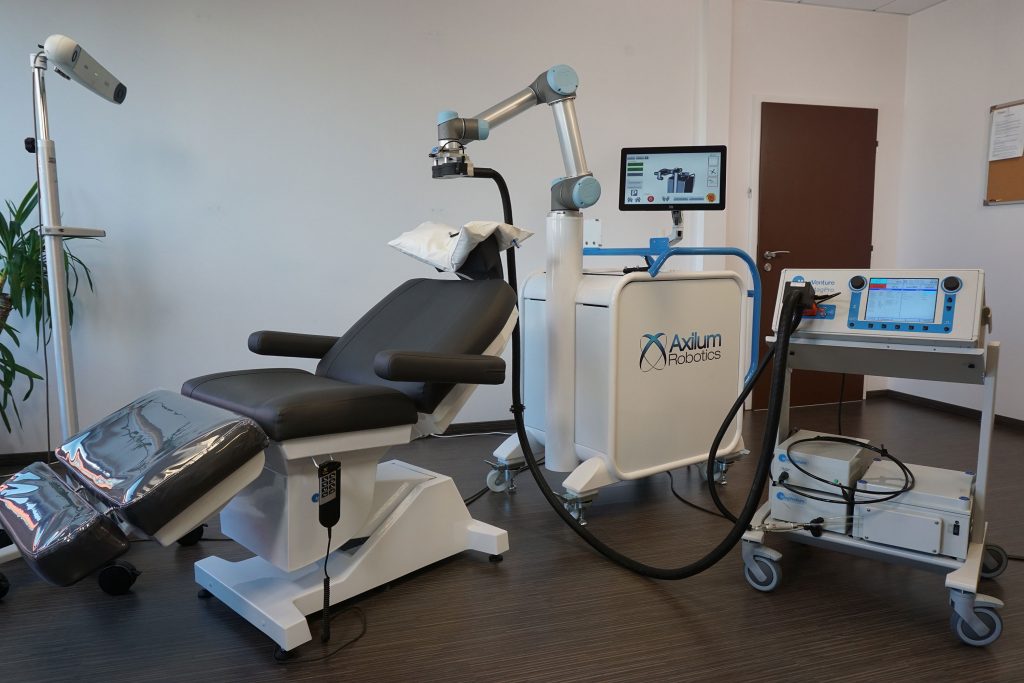
Read MoreAxilum Wins 510(k) Clearance for Robotic TMS System
Axilum Robotics, which specializes in the development of medical robots, announced that two weeks after the CE mark, it has received 510(k) clearance from the U.S. Food and Drug Administration to market the TMS-Cobot TS MV for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system. The company successfully developed…
Read MoreIntuitive Surgical Gains FDA Clearance for Lung Cancer Biopsy Robot
Intuitive Surgical Inc. said last week that the U.S. Food and Drug Administration has cleared its Ion system. The robotic endoluminal system is designed to enable doctors to conduct minimally invasive biopsies deep within the lung. Ion includes an articulating robotic catheter with an outer diameter of only 3.5 mm that is able to move…
Read MoreJ&J Acquires Robotic-Surgery Firm Auris for $3.4 Billion
Focused on creating the next frontier of surgery, Johnson & Johnson, today announced that Ethicon, Inc., entered into a definitive agreement to acquire Auris Health, Inc. for approximately $3.4 billion in cash. Additional contingent payments of up to $2.35 billion, in the aggregate, may be payable upon reaching certain predetermined milestones. Auris Health is a…
Read MoreNorthwestern Memorial Hospital Performs First US Robotic Lung Volume Reduction Surgery
Northwestern Memorial Hospital is the first in the country to successfully complete robotic-assisted lung volume reduction surgery (LVRS) using the da Vinci Xi Surgical System® to remove diseased, emphysematous tissue within the lungs for the treatment of severe emphysema, a progressive form of chronic obstructive pulmonary disease (COPD). The robotic procedure allows the surgeon to precisely remove the…
Read MoreTransEnterix Wins FDA Clearance for Senhance Ultrasonic Instruments
TransEnterix, Inc., a medical device company that is digitizing the interface between surgeons and patients to improve minimally invasive surgery, today announced the Company received FDA 510(k) clearance for its Senhance Ultrasonic System. “Advanced energy devices are used within a high percentage of cases across a wide range of procedures, which make them a critical tool for…
Read More