Diagnostics & Healthcare News
empowerDX At-Home COVID-19 Testing Kit Receives EUA For Children Three Years And Older
Clinical Enterprise, Inc. d/b/a empowerDX, today announced its at-home COVID-19 testing kit received FDA-emergency use authorization for children three years and older. The direct-to-consumer company, owned by Eurofins Scientific, is the first to receive an EUA for an at-home PCR nasal test for young children. empowerDX launched its FDA-authorized at-home COVID test in the fall of 2020…
Read MoreNeurolutions Receives FDA De Novo Market Authorization for IpsiHand Robotic Stroke Rehab System
Neurolutions, Inc., a medical device company developing and commercializing a first-of-its-kind device leveraging brain-computer interface (BCI) technology for upper extremity chronic stroke rehabilitation, announced today that the U.S. Food and Drug Administration (FDA) granted De Novo market authorization for its groundbreaking IpsiHand Upper Extremity Rehabilitation System. The IpsiHand System has been cleared for use in…
Read MoreSmiths Medical and Ivenix Partner to Revolutionize Infusion Management with First-Ever Comprehensive Suite of Infusion Solutions in US Healthcare Market
Bolstered by strategic investment from Smiths Medical, commercial partnership will offer best-in-class infusion systems designed to improve patient safety, advance clinical efficiency Smiths Medical, a global medical device leader, today announced it has formed an exclusive partnership with Ivenix, Inc. that positions the companies as the first in the U.S. to offer a comprehensive suite…
Read MoreQorvo Biotechnologies Receives FDA Emergency Use Authorization for Rapid COVID-19 Antigen Testing
Qorvo, a leading provider of innovative radio frequency (RF) solutions that connect the world, today announced that the U.S. Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for the Qorvo Omnia™ SARS-CoV-2 Antigen Test. The test is authorized for the qualitative detection of nucleocapsid viral antigens from SARS-CoV-2 in nasal swab specimens…
Read MoreZOLL Medical Corporation Acquires Respicardia
ZOLL Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, today announced that it has acquired Respicardia, Inc., a provider of novel implantable neurostimulators for the treatment of moderate to severe Central Sleep Apnea (CSA). CSA is a serious condition that is often associated with heart failure, coronary artery disease,…
Read MoreDonisi Wins De Novo Clearance for Contact-Free AI System that Spots Changes in Heart, Breathing Patterns
The U.S. Food and Drug Administration (FDA) has granted Donisi‘s revolutionary contact-free multiparameter measurement system de novo clearance, making Donisi the first to bring this innovative technology to the medical market. “I’m really proud of our team; we developed a medical device that can change the lives of millions of people. Our novel technology is now recognized and…
Read MoreOpSens Announces Closing of $28.75 Million Bought Deal Public Offering Including $3.75 Million Over-Allotment Option Exercised in Full
OpSens Inc. (“OpSens” or the “Company”) (TSX:OPS) (OTCQX:OPSSF) announced last week the closing of its previously announced bought deal public offering (the “Offering”) of common shares of the Company (the “Common Shares”), for total gross proceeds of approximately $28,750,000. The Company issued an aggregate of 15,972,222 Common Shares, at a price of $1.80 per Common Share,…
Read MoreFDA clears Signifier Medical’s Electric Tongue Muscle Strengthener to Treat Sleep Apnea and Snoring
The FDA has approved eXciteOSA, the revolutionary first-ever daytime treatment for mild obstructive sleep apnea and snoring. Used for only 20 minutes per day for a period of six weeks and then twice per week, the therapy is clinically proven to improve the quality of sleep by significantly reducing obstructive sleep apnea and snoring. Signifier…
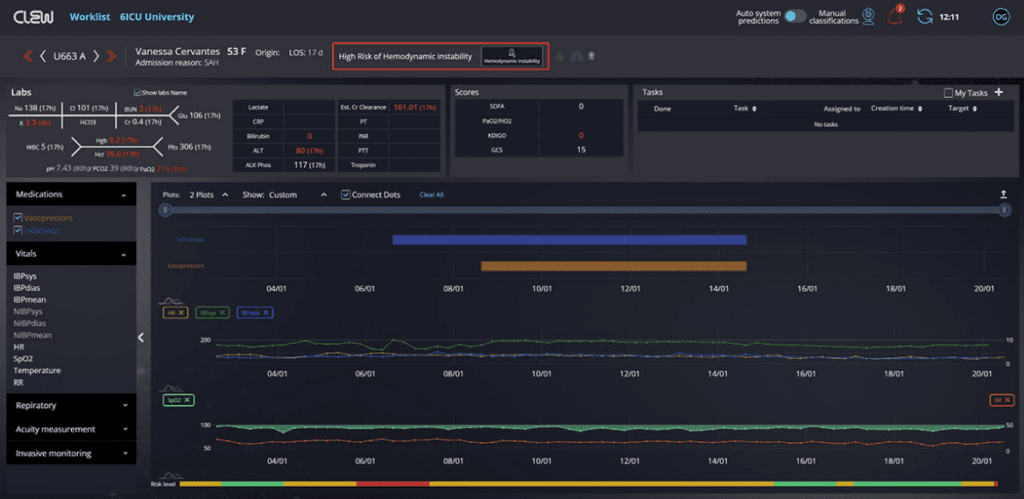
Read MoreCLEW Medical Receives FDA Clearance for AI-Based Software that Predicts Hemodynamic Instability
CLEW Medical, a leader in AI-powered predictive analytics, today announced that the U.S. Food and Drug Administration (FDA) has given 510(k) clearance and authorized the use of “CLEWICU,” CLEW’s artificial intelligence (AI) based ICU solution, to predict hemodynamic instability in adult patients. The clearance is the FDA’s first for such a device, and follows the…
Read MoreInovytec’s Small “Sparrow” Ventilators Receive FDA Clearance
Inovytec, an innovator of multi-functional and user-friendly critical medical devices, announced today that it was granted FDA 510(k) clearance to market and sell its Ventway Sparrow ventilators in the United States. The ventilators are already commercialized in Europe, Canada and Australia and undergoing registration procedures in other countries. The Ventway Sparrow transport and emergency ventilators are designed for mobility, high-performance…
Read More