Diagnostics & Healthcare News
AdvaMed Commends Proposed CMS Rule to Cover “Breakthrough” Medical Devices
Following today’s issuance by the Centers for Medicare and Medicaid Services (CMS) of a proposed rule on Medicare coverage of innovative technologies, the Advanced Medical Technology Association (AdvaMed) issued the following statements from President and CEO Scott Whitaker and other medtech industry leaders commending the agency’s action: “In order to incentivize innovative medical breakthroughs, the…
Read MoreSynchron’s Stentrode Brain-Computer Interface Receives Breakthrough Device Designation from FDA
Synchron, a neurovascular bioelectronics medicine company, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation for Stentrode, a fully-implantable medical device that can translate brain activity or stimulate the nervous system from the inside of a blood vessel, without the need for open brain surgery. The device, which has…
Read MoreValencia Technologies Files Pre-Market Approval Application with U.S. FDA for eCoin Peripheral Neurostimulator
Valencia Technologies Corporation (“Valencia“), a private medical device company, today announced the filing of its pre-market approval (PMA) application of its eCoin® Peripheral Neurostimulator System for overactive bladder with the Food and Drug Administration (FDA). eCoin® is a first-in-kind neurostimulator providing intermittent electrical stimulation of the tibial nerve via a leadless nickel-sized and shaped device fully-implanted under…
Read MoreCeek Women’s Health Introduces Nella NuSpec, a Smarter Reusable Vaginal Speculum
Ceek Women’s Health, a groundbreaking women-led medical device company, is excited to announce its latest innovation, the Nella NuSpec Reusable Vaginal Speculum. The Nella NuSpec was developed in partnership with OBGYN providers and patients through an extensive 5-year R&D process. The reusable vaginal speculum commonly used today is made from metal and has not changed…
Read MoreMagVenture Receives FDA Clearance for OCD
FDA has cleared MagVenture TMS Therapy for adjunct treatment of Obsessive-Compulsive Disorder (OCD). This marks the second indication in the US for the Danish medical device company MagVenture who is already FDA cleared for the treatment of major depressive disorder. OCD is a mental health disorder characterized by unreasonable thoughts and fears (obsessions) which lead…
Read MoreNeurolief Wins FDA Breakthrough Device Designation for Wearable Neuromod for Depression
Neurolief, a medical neurotechnology innovator, today announces that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation to the Relivion system, the first non-invasive multi-channel brain neuromodulation technology, for the treatment of major depression. A wearable device, the Relivion system is designed as an adjunctive treatment to pharmaceutical management of major depressive disorder…
Read MoreeSight 4 Earns CE Mark Approval to Bring New Wearable Device to Europe
eSight, a cutting-edge vision enhancement platform, today announces it has received CE Mark approval for eSight 4, the company’s latest assistive technology device that’s clinically proven to significantly enhance vision for those living with low vision and legal blindness. The CE Mark certification signifies that eSight 4 has been assessed to meet high safety, health…
Read MorePhilips Wins FDA Clearance for EMS Remote Monitoring and Defibrillation Solution
Royal Philips, a global leader in health technology, today announced the launch of its remote monitoring and defibrillator solution (Tempus ALS) for pre-hospital settings in the U.S. The solution is a complete end-to-end system that combines innovative hardware and advanced software to expand the pre-hospital scope of care for first responders. The professional defibrillator (Tempus…
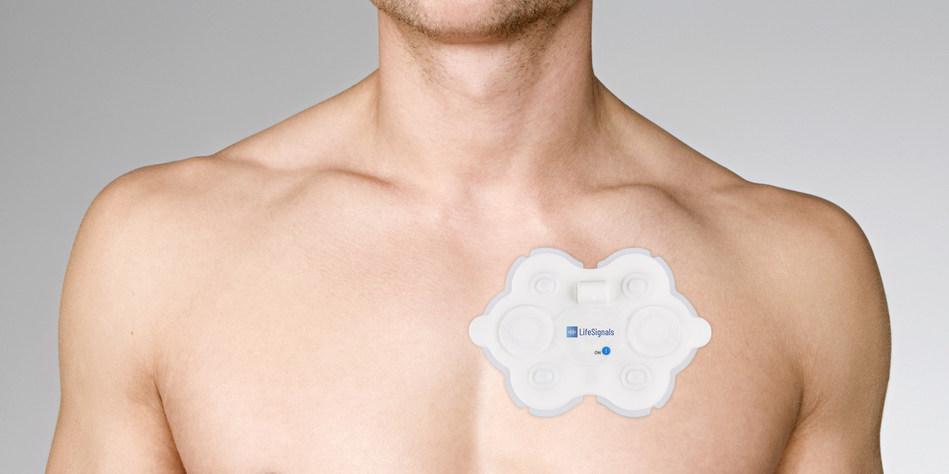
Read MoreLifeSignals Wireless Medical Biosensor Receives FDA Clearance
LifeSignals Group, today announced USFDA 510(k) clearance has been received for their LifeSignals ECG Remote Monitoring Patch Platform. The LifeSignals ECG Remote Monitoring Patch Platform is a wireless remote monitoring system intended for use by healthcare professionals for the continuous collection of Electrocardiography (ECG) and Heart Rate monitoring in ambulatory, hospital, home and healthcare settings.…
Read MoreUK Watchdog Skeptical of Stryker’s $4B Wright Medical Dea
The U.K. Competition and Markets Authority on Tuesday raised concerns that Stryker’s proposed $4 billion acquisition of Wright Medical Group could result in the medtech giant controlling 90% of Britain’s total ankle replacement prostheses market, leading to higher prices and less choice for hospitals and patients. CMA warned that the anti-competitive nature of the Stryker-Wright deal could have a negative…
Read More