Imaging Visualization & Navigation
Philips and Insightec Teaming Up on Ultrasound for Neurosurgery
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology and InSightec, a global healthcare company focused on the therapeutic power of acoustic energy, today announced a collaboration to expand access to MR-guided focused ultrasound for incisionless neurosurgery. By developing compatibility between Philips’ advanced MR systems and the Exablate Neuro platform from InSightec, the two…
Read MoreSiemens X-Ray System Gains FDA Clearance
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the YSIO X.pree, a ceiling-mounted radiography system with the MyExam Companion intelligent user interface. In addition to this easy-to-use interface that guides the radiologic technologist through the exam workflow, the YSIO X.pree has a new 3D camera for patient positioning and advanced collimation,…
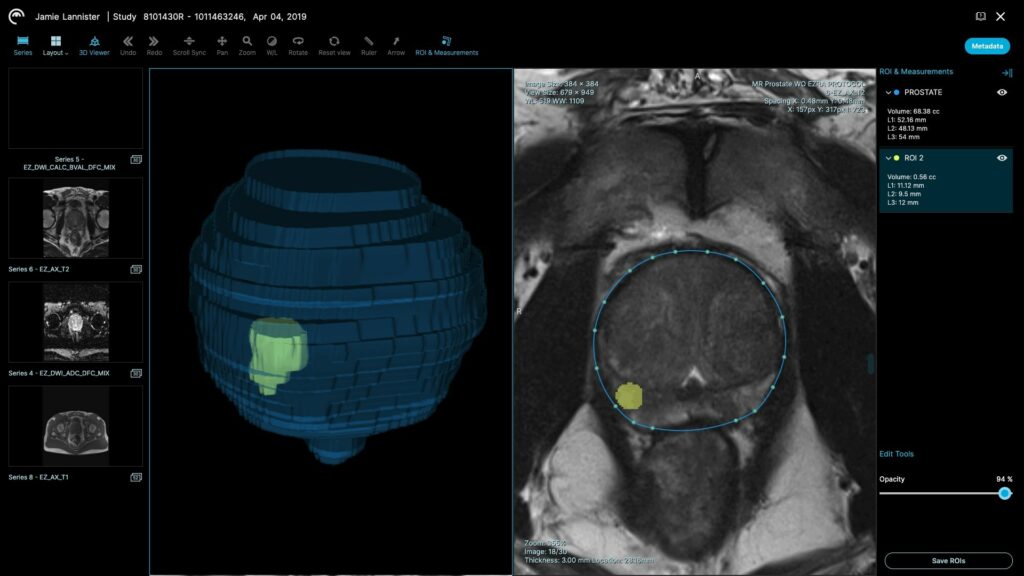
Read MoreEzra Receives FDA Clearance for Prostate Cancer Artificial Intelligence
Ezra, the New York-based startup transforming early cancer screening using MRI, announced today that it has received FDA 510(k) clearance for its Artificial Intelligence designed to assist radiologists in analyzing and segmenting prostate MRI. Use of the innovative AI technology can help reduce the time and cost of MRI-based prostate cancer screening. It is the first…
Read MoreFDA Grants GI Windows Medical Breakthrough Device Designation for Magnetic Anastomosis Technology
GI Windows Medical Corp, a clinical-stage, privately-held medical device company pioneering new advancements in anastomosis technology announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the self-forming magnetic compression anastomosis device indicated for small bowel end to end anastomosis for ileostomy reversal or tissue resection. The Breakthrough Devices Program is…
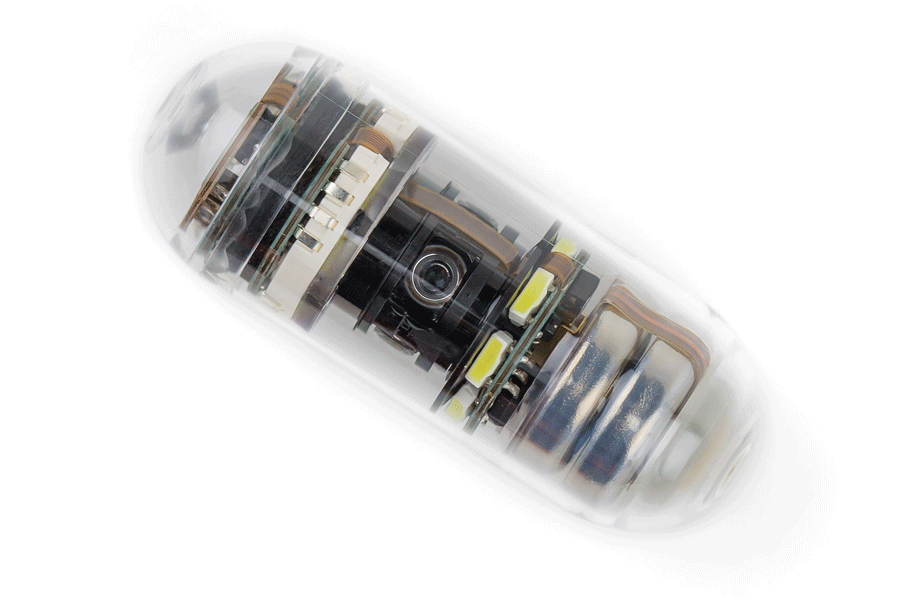
Read MoreFDA Allows At-Home Administration for CapsoVision Capsule Endoscope
CapsoVision, an innovator in the gastroenterology diagnostics market, today announced that the U.S Food & Drug Administration (FDA) will apply enforcement discretion which allows at-home administration of the CapsoCam Plus® small bowel capsule endoscope during the COVID-19 pandemic for patients who are determined eligible for at-home administration. The labeling addendum permits a fully remote capsule endoscopy…
Read MoreNOUS Imaging Receives 510(k) Clearance from FDA for Real Time MRI Software
NOUS Imaging, a medical imaging software company, today announced that the FDA has cleared its 510(k) submission for FIRMM, Framewise Integrated Real-Time MRI Monitoring. FIRMM provides real-time monitoring and biofeedback that addresses the significant problem of patient motion during brain MRI. This is the Company’s first FDA clearance and represents the initial piece of a…
Read MoreFujifilm Sonosite Receives FDA COVID-19 510(k) Clearance for Point-of-Care Ultrasound Portfolio
FUJIFILM Sonosite, Inc., specialists in developing cutting-edge point-of-care ultrasound (POCUS) solutions, and part of the larger Fujifilm Healthcare portfolio, today announced that the company has received 510(k) Clearance from the U.S. Food & Drug Administration (FDA) for the company’s entire POCUS portfolio to support healthcare providers in performing accurate lung and cardiac imaging in COVID-19 patients. Fujifilm…
Read MoreHyperfine Receives FDA 510(k) Clearance for Portable MRI System
Hyperfine Research, Inc. has received 510(k) clearance from the US FDA for its category-defining portable MRI technology, the Swoop™ Portable MR imaging device. Hyperfine’s Swoop™ system is a point-of-care MR imaging device that wheels directly to the patient’s bedside, plugs into a standard electrical wall outlet and is controlled through a wireless tablet, making MR imaging…
Read MoreSiemens Healthineers to buy Varian Medical Systems for $16.4 billion
Varian today announced that it has entered into a definitive agreement to combine with Siemens Healthineers AG in an all-cash transaction valued at $16.4 billion on a fully diluted basis. Under the terms of the agreement, which has been unanimously approved by Varian’s Board of Directors, Siemens Healthineers will acquire all outstanding shares of Varian…
Read MoreFDA Clears Zebra Medical’s Breast Cancer AI for Spotting Suspicious Mammography Lesions
Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its sixth FDA 510(k) clearance for its mammography solution, HealthMammo, which has already received a CE mark. Zebra Medical’s algorithm empowers breast radiologists by prioritizing and identifying suspicious mammograms, providing a safety net for radiologists. The suspicious mammograms are identified faster and read earlier…
Read More