Imaging Visualization & Navigation
Echosens Launches FibroScan GO, Affordable State-of-the-Art, Non-Invasive Solution for Identifying Patients with Advanced Chronic Liver Disease
Echosens, a high-technology company offering the FibroScan® portfolio of solutions, is pleased to announce the launch of FibroScan GO, an affordable, cost-effective tool for screening liver health, improving liver health management at the point of care for primary care practices and reducing the flow of patients in secondary care centers. FibroScan GO is a solution for…
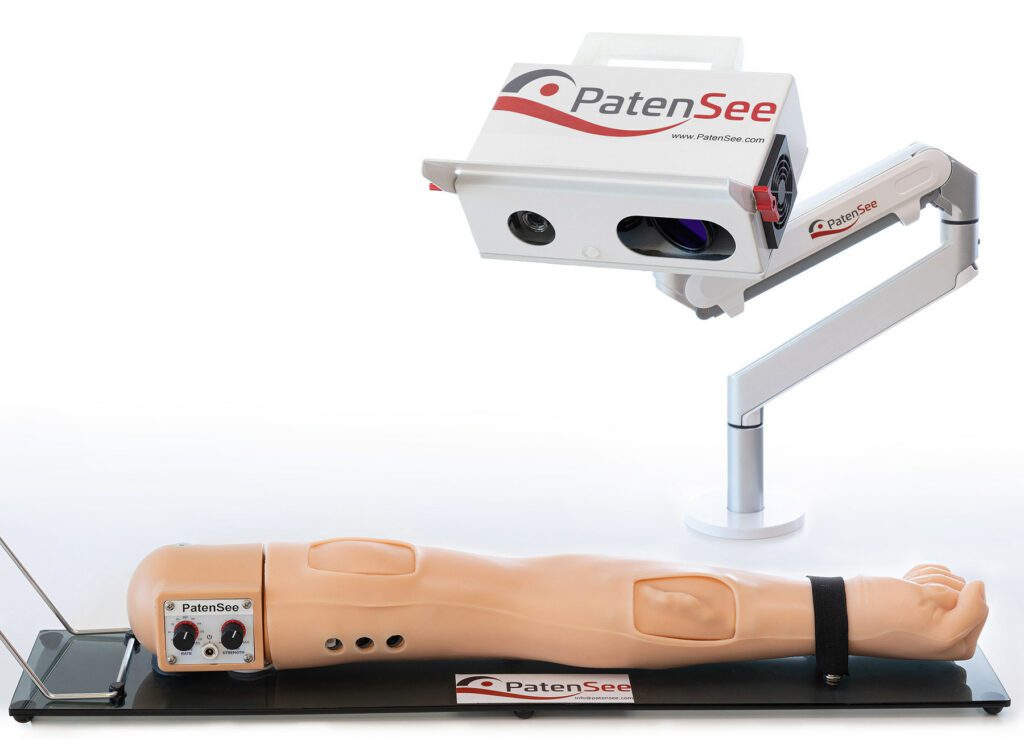
Read MorePatenSee’s Contactless Imaging Device Shows Promise in Monitoring Dialysis Patients
The interim results of an ongoing clinical study of PatenSee‘s contactless imaging device for the early detection of vascular access stenosis for dialysis patients were shared in a poster presentation. The presentation, titled First Clinical Experience with Non-Invasive, Contactless, Optical Surveillance of Vascular Access, was delivered by the study’s principal investigator, Professor Benaya Rozen-Zvi, MD at the 2022 World…
Read MoreLatest iCad 3D Mammography AI Gains CE Mark
iCAD, Inc., a global medical technology leader providing innovative cancer detection and therapy solutions, today announced that ProFound AI® Version 3.0 for Digital Breast Tomosynthesis (DBT) received CE Mark approval. Compared to previous software versions, the latest generation of ProFound AI offers up to a 10% improvement in specificity performance while maintaining an industry-leading high sensitivity…
Read MoreActiv Surgical Announces FDA Clearance for ActivSight Enhanced Surgical Visualization Device
Activ Surgical, a digital surgery pioneer, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance of the company’s ActivSight Intraoperative Imaging Module for enhanced surgical visualization. The hardware agnostic imaging module has been designed to provide surgeons real-time intraoperative visual data and imaging not currently available to surgeons through existing technologies, helping to improve…
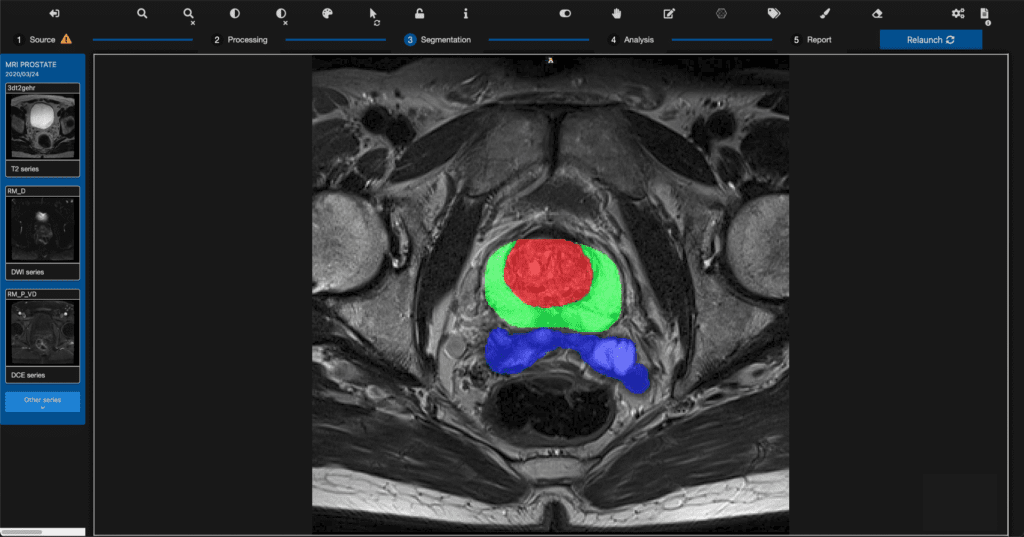
Read MoreQuibim Receives FDA 510(k) Clearance for qp-Prostate AI Solution for Prostate MRI Analysis
Quibim, a global leader in whole-body medical imaging analysis, announced today the launch of qp-Prostate, its latest and most advanced prostate AI based Magnetic Resonance (MR) solution, after receiving 510(k) clearance by the US Food and Drug Administration. The solution aids in the process of prostate magnetic resonance imaging (MRI) reporting from visualization to quantification with…
Read MoreZAP Surgical Receives CE Mark Clearance for its ZAP-X Gyroscopic Radiosurgery Platform
ZAP Surgical Systems, Inc. today announced it has received CE mark clearance to the new EU MDR for its ZAP-X® Gyroscopic Radiosurgery® platform. The CE mark makes it possible for healthcare providers to begin treating patients with the ZAP-X system in the European Union. For select indications including many primary and metastatic brain tumors, stereotactic…
Read MoreFDA Clears MULTIX Impact C Ceiling-Mounted DR System
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the MULTIX Impact C ceiling-mounted digital radiography (DR) system as well as the MULTIX Impact VA20, a new version of the established floor-mounted parent DR system. Economically priced, both systems expand access to high-quality imaging and enhance the patient experience. The MULTIX Impact…
Read MoreFDA Approves Seno Medical’s Breast Lesion Diagnostic Technology
The Center for Devices and Radiological Health (CDRH) of the US Food & Drug Administration (FDA) has granted Texas-based Seno Medical Instruments, Inc. (Seno) premarket approval (PMA) for its groundbreaking diagnostic breast cancer imaging technology that helps physicians better differentiate between benign and malignant breast lesions. The company’s Imagio Breast Imaging System uses non-invasive opto-acoustic ultrasound…
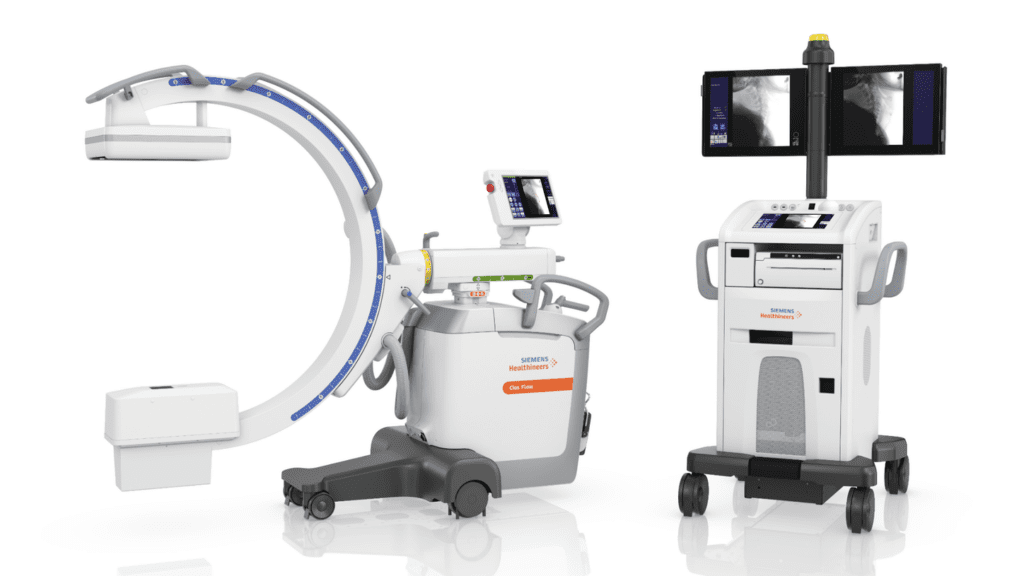
Read MoreFDA Clears Siemens’ Cios Flow Mobile C-arm System
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the Cios Flow, a mobile C-arm designed for use by multiple disciplines in the operating room (OR) – including orthopedics, trauma surgery, spinal surgery, vascular surgery, and pain therapy – to increase the ease and efficiency of everyday imaging workflows for surgical interventions.…
Read MoreHologic to Acquire Somatex Biopsy Site Marker Technology for $64 Million
Hologic (NSDQ:HOLX) announced today that it completed the acquisition of Somatex Medical Technologies GmbH for $64 million. Marlborough, Mass.-based Hologic’s acquisition of the company, which was previously owned by E-Med Solutions GmbH, is slated to support its strategy of providing further solutions across the continuum of breast health care and across Europe, according to a news release. Somatex’s…
Read More