Orthopedics and Spine
Life Spine Announces FDA 510(k) Clearance of the Titanium Stand-Alone ALIF Spacer System
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the Titanium Stand-Alone ALIF Spacer System. “This system clearance is the capstone on a year of incredible product…
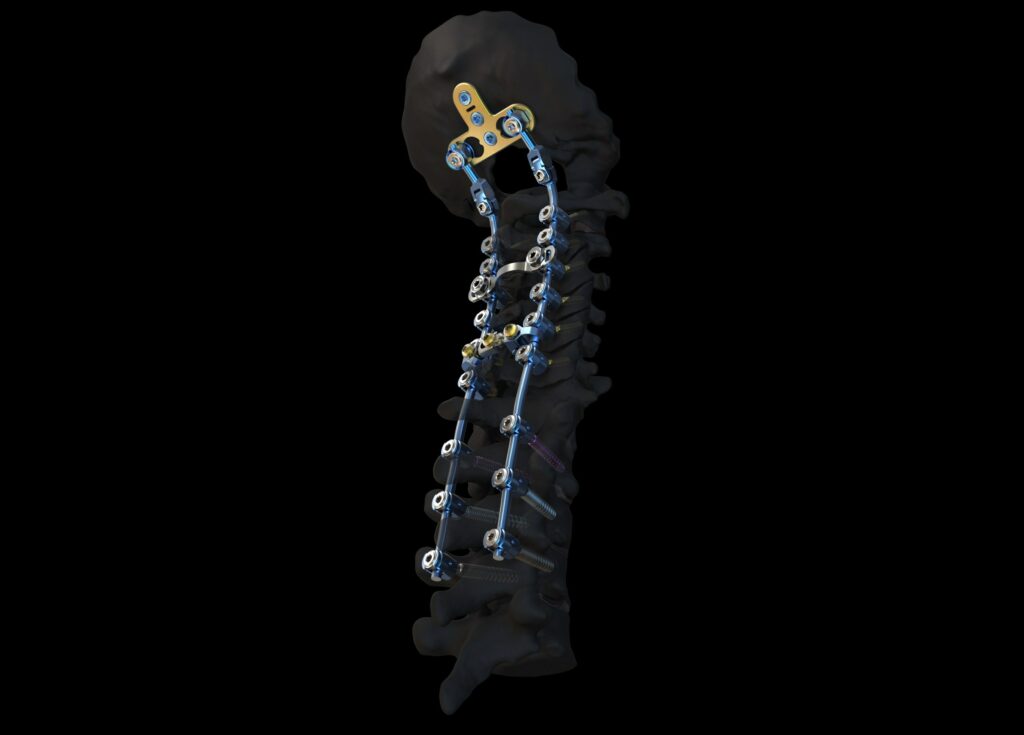
Read MoreNew Cervical Spine System from DePuy Synthes Advances Treatment Options for Patients with Complex Cervical Spine Disorders
The Johnson & Johnson Medical Devices Companies* today announced that DePuy Synthes has launched the SYMPHONY Occipito-Cervico-Thoracic (OCT) System, expanding its offering for the surgical treatment of conditions in the neck and upper back. The SYMPHONY System includes a differentiated offering of instruments and implants designed for stabilization of the spine in patients undergoing Posterior…
Read MoreWorld’s First “Artificial Meniscus” Available in Israel
Active Implants LLC, a company that develops orthopedic implant solutions, today announced that two patients in Israel have undergone knee surgery for the company’s NUsurface® Meniscus Implant – the first “artificial meniscus” to be marketed in the Middle East. Until now, the NUsurface Implant was only available in Israel in clinical trials. The procedures were performed…
Read MoreNevro’s Senza Omnia Spinal Cord Neurostimulation System Gains FDA Approval
Nevro Corp. (NYSE: NVRO), a global medical device company that provides innovative, evidence-based solutions for the treatment of chronic pain, today announced it has received approval from the U.S. Food and Drug Administration (FDA) for the Senza® Omnia™ Spinal Cord Stimulation (SCS) System. The Omnia system is the first and only SCS system designed to deliver Nevro’s…
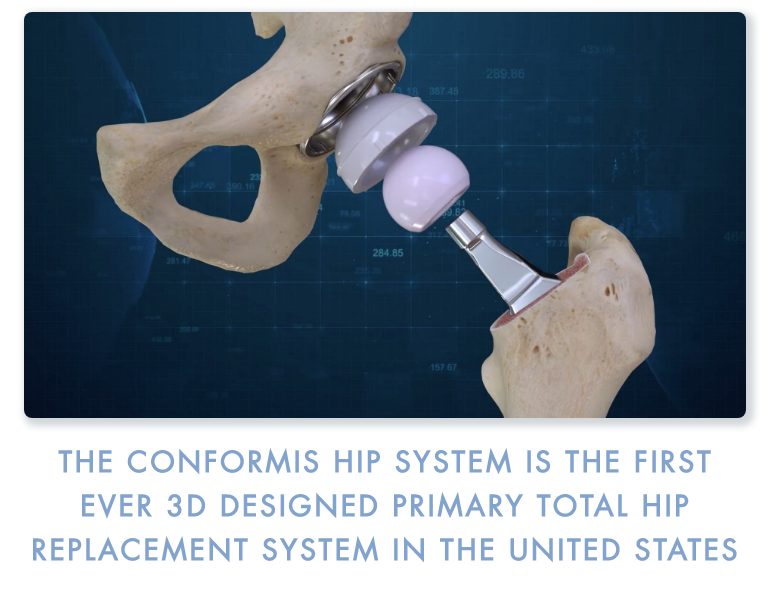
Read MoreConformis Announces FDA Clearance and Full Commercial Launch of Next Generation Hip System
Conformis, Inc. (NASDAQ:CFMS), a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture, and sell patient-specific arthroplasty joint replacement implants designed to fit each patient’s unique anatomy, today announced FDA clearance of the Company’s next generation Conformis Hip System. The Company will be featuring the new 3D-designed Conformis Hip System as…
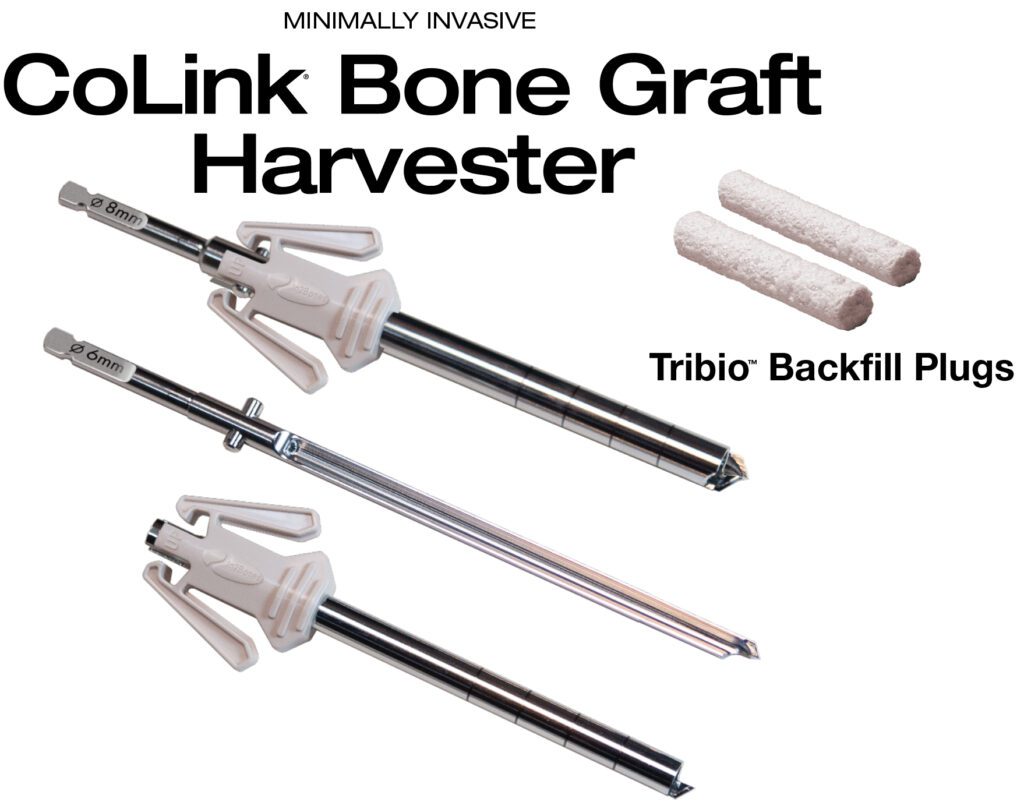
Read MoreIn2Bones Introduces CoLink Bone Graft Harvester And Tribio Backfill Plugs
In2Bones Global, Inc. today announced the U.S. commercial launch of its CoLink® Bone Graft Harvester and Tribio™ Backfill Plugs System. Packaged sterile and pre-assembled for single use, the CoLink Bone Graft Harvester is a minimally invasive bone graft device that harvests bone from various sites in the body, including the calcaneus, iliac crest, proximal tibia, distal tibia, and distal femur.…
Read MoreDiFusion Inc. Announces FDA 510K Clearance for its Spinal Interbody device, the Xiphos ZFUZE
DiFusion, Inc. today announced the FDA 510K clearance for the Xiphos-ZF Spinal interbody device. Xiphos-ZF is the first spinal implant developed from a new biomaterial called ZFUZE. Multiple studies have shown the ZFUZE material elicits pro-reparative M2 macrophage response and significant reductions in Interleukin 1-Beta and Interleukin 6. IL1-Beta and IL6 are cytokine markers for…
Read MoreADAM 3D Bone Printing Project Promises to Change How We Think About Our Bodies
ADAM, a 3D bone printing project that has already secured partnerships with ARMI, Stanley Black & Decker, and Big Pharma, is weeks away from launching the first clinical trials. But the project’s ambitions reach much further than simply creating bones. What differentiates ADAM from similar biotech projects is its innovations in speed and precision, as…
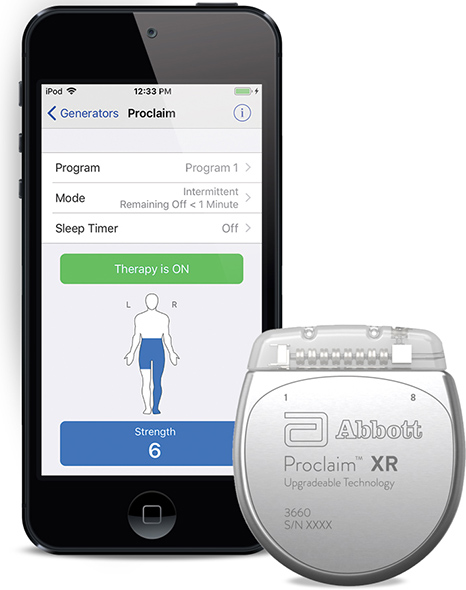
Read MoreFDA Approves Abbott’s “Low Dose,” Recharge-Free Spinal Cord Stimulation System with up to Ten Year Battery Life
Abbott announced the U.S. Food and Drug Administration (FDA) has approved the company’s Proclaim XR™ recharge-free neurostimulation system for people living with chronic pain. The Proclaim XR platform offers a low dose of Abbott’s proprietary BurstDR™ stimulation waveform, which was created based on scientific insights from doctors and research to mimic natural patterns found in…
Read MoreCentinel Spine Announces IDE Approval of Two Different Prodisc C Devices
Centinel Spine said earlier this week that it won an investigational device exemption from the FDA for a clinical trial of its Prodisc cervical disc implants. New York City-based Centinel acquired the Prodisc assets from Johnson & Johnson subsidiary DePuy Synthes in December 2017 for an undisclosed amount. The FDA IDE covers a two-level study of the company…
Read More