Diagnostics & Healthcare News
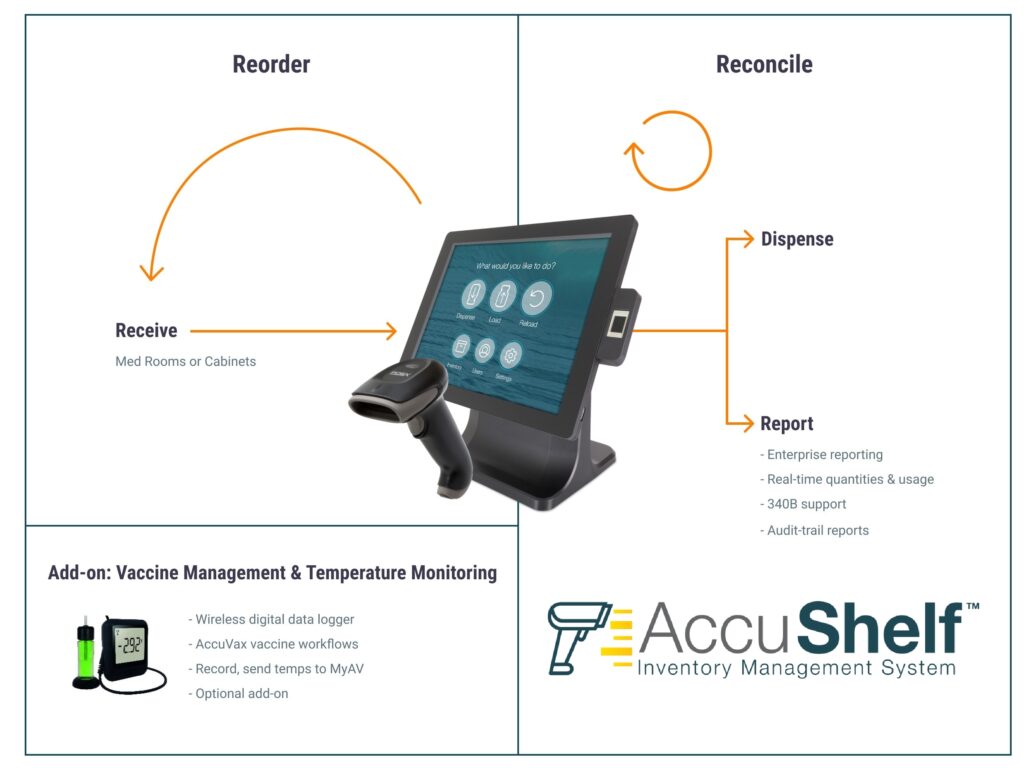
TruMed Systems, Inc. Announces Launch of the AccuShelf Total Practice Inventory Management System, to Complement the AccuVax Vaccine Storage and Handling Solution
AccuShelf makes inventory control simple, from tracking of receiving items, to patient administration. The system utilizes an all-in-one touch screen PC system with an integrated biometric reader and a wireless barcode scanner to capture and record every medication dose with lot, and expiration. It tracks each dose to a specific patient and can communicate with…
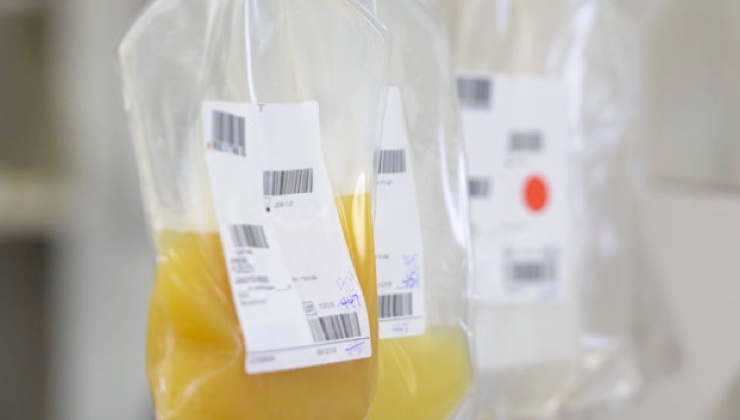
Read MoreCerus Corporation Announces FDA Approval for INTERCEPT Blood System for Plasma with Alternate Plastic Disposable Kits
Cerus Corporation (Nasdaq:CERS) announced today FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative plastic disposable kit. The planned conversion to these new kits is part of the Company’s ongoing strategy to enhance its global supply chain integrity that was initiated several years ago. “The FDA approval for the new INTERCEPT plasma…
Read MoreMolekule Launches New Commercial Product, Air Pro RX, with FDA 510(k) Class II Medical Device Clearance
Molekule, the leader in reinventing air purification, today announced that the U.S. Food and Drug Administration (FDA) cleared the 510(k) premarket notification for its new medical-grade air purifier, Air Pro RX, classifying it as a 510(k) Class II Medical Device. The Molekule Air Pro RX air purifier is intended for medical purposes to destroy bacteria and viruses in…
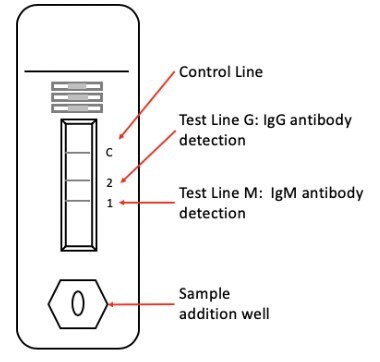
Read MoreZEUS Scientific Announces New Rapid SARS-CoV-2 Antibody Test
ZEUS Scientific announces today the submission for an Emergency Use Authorization (EUA) to the U.S. Food and Drug Administration (FDA) for its rapid, in vitro diagnostic test for the qualitative detection of IgG and/or IgM antibodies to the SARS-CoV-2 (novel 2019 Coronavirus). ZEUS’s lateral flow test uses patient serum, plasma or whole blood and provides results in…
Read MoreNew Renewable Energy Agreements To Reduce Boston Scientific Carbon Footprint By Half
Boston Scientific Corporation (NYSE: BSX) has signed a virtual power purchase agreement (VPPA) that represents the largest step the company has taken to achieve its goal of global carbon neutral manufacturing and distribution by 2030. The agreement, with Clearway Energy Group, will address the electricity load for the company’s U.S. operations, which represents 45 percent of…
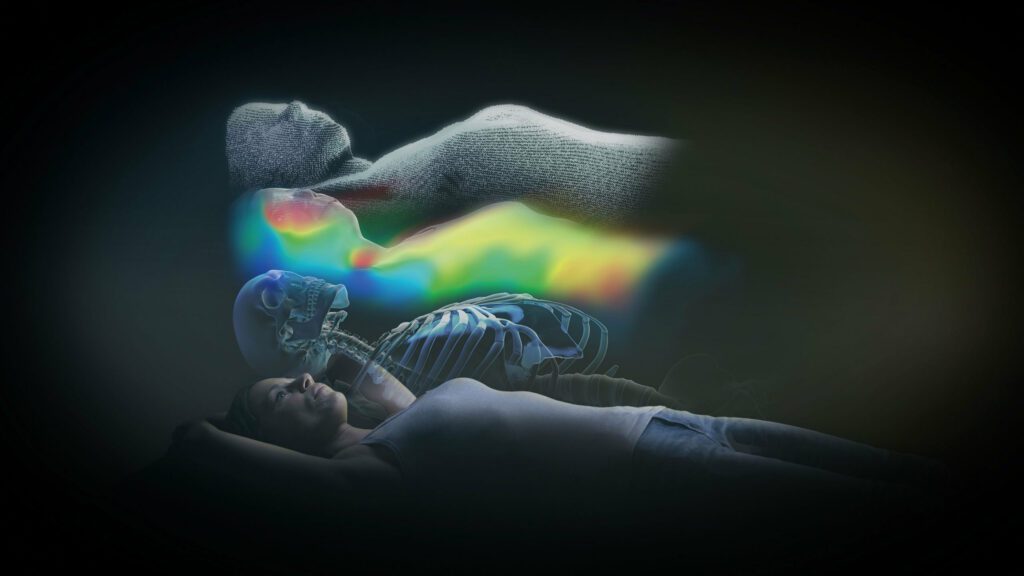
Read MoreBrainlab Announces CE Mark for ExacTrac Dynamic Patient Positioning and Monitoring
Brainlab, the digital medical technology company, today announced CE Mark (Conformité Européenne) approval for ExacTrac® Dynamic, the company’s next generation patient positioning and monitoring system. The new system offers never-before-seen, high-speed thermal surface tracking technology combined with an update of ExacTrac X-ray monitoring, providing advanced capabilities. New clinical workflows allow for treatment of a wide array of…
Read MoreVent Multiplexor Receives FDA Emergency Use Authorization for Crisis Care Co-Ventilation During COVID-19 Pandemic
Vent Multiplexor, LLC and Yale New Haven Hospital announced today that the Food and Drug Administration has granted Emergency Use Authorization for the Vent Multiplexor, a life-saving emergency rescue device developed by Vent Multiplexor LLC in collaboration with Yale New Haven Hospital. The Vent Multiplexor is a patent-pending device designed to provide individualized emergency crisis…
Read MoreFDA Encourages Recovered Patients to Donate Plasma for Development of Blood-Related Therapies
As part of the all-of-America approach to fighting the COVID-19 pandemic, the U.S. Food and Drug Administration has been working with partners across the U.S. government, academia and industry to expedite the development and availability of critical medical products to treat this novel virus. Today, we are providing an update on one potential treatment called…
Read MoreFDA Grants Breakthrough Device Designation to DNAe’s Sequencing Diagnostic
DNAe, the next generation sequencing company developing novel diagnostics for use at the point-of-need, today announced that the US Food and Drug Administration (FDA) has granted it a “Breakthrough Device” designation for its pioneering platform and first assay. DNAe has reinvented sequencing in order to develop a compact device operable by non-specialist users. LiDia-SEQ™ will,…
Read MoreSpiderTech Helping Healthcare Workers Experiencing Irritation, Sores From PPE With Face Protection
In the midst of the COVID-19 pandemic, healthcare workers across the world have been active on social media displaying the painful results of wearing medical personal protective equipment (PPE) for long hours on the job. Face masks, surgical and N95 masks, eyewear, facial hoods and other protective measures have resulted in red, sore, irritated skin…
Read More