Diagnostics & Healthcare News
Stryker Releases Emergency Relief Bed, a Limited-Release Medical Bed to Support Critical Needs During Pandemic
Stryker, one of the world’s leading medical technology companies, announced today it has developed a low-cost, limited-release emergency response bed to quickly aid healthcare providers with efficient care during the COVID-19 pandemic. “People are at the heart of what we do, and COVID-19 hasn’t changed that. It has amplified our mission of making healthcare better.…
Read MoreCOVID-19 Antibody Test Released by Ortho Clinical Diagnostics
Aligned with its mission to improve and save lives with diagnostics, Ortho Clinical Diagnostics today announced it is launching to market its SARS-CoV-2 (COVID-19/coronavirus) antibody test—the VITROS® Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack. Testing kits are expected to be available in a few weeks. Ortho followed the guidelines established by the U.S. Food and Drug Administration’s…
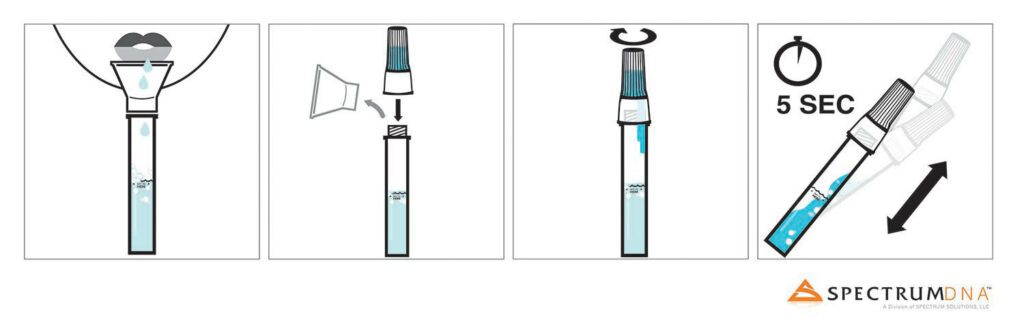
Read MoreSpectrum Solutions Saliva Collection Device Answers Critical Testing Hurdles, Enabling Large-Scale COVID-19 Testing While Protecting Health Professionals From Exposure
Spectrum DNA™, a division of Spectrum Solutions, LLC, in Salt Lake City, Utah, today announced that using the Spectrum DNA SDNA-1000 Whole Saliva Collection Device, researchers from RUCDR Infinite Biologics at Rutgers University have successfully validated saliva as being a viable biosample source for COVID-19 detection when compared to nasopharyngeal or oropharyngeal swabs. The resourceful…
Read MoreTablo Hemodialysis System Receives FDA Clearance for Home Dialysis
Outset Medical, a leading med tech innovator delivering first-of-its-kind technology into the growing global dialysis market, today announced the Food and Drug Administration (FDA) cleared the Tablo Hemodialysis System for patient use in the home. The new home clearance expands Tablo’s existing labeled indication for use in acute and chronic care facilities, which for the…
Read MoreCrossBay Medical Receives FDA Clearance for CrossGlide Endometrial Tissue Sampler
CrossBay Medical, Inc., a health technology company focused on improving the delivery of women’s healthcare, announced it has received clearance from the Food and Drug Administration (FDA) to market its CrossGlide™ ETS Endometrial Tissue Sampler. The CrossGlide ETS, the third product to utilize the frictionless CrossGlide technology platform, enables medical providers to perform an office-based…
Read MoreNuvo Group Receives FDA Clearance for its Innovative INVU Remote Pregnancy Monitoring System
Nuvo Group, a private company with a bold ambition to reinvent pregnancy care for the 21st century, today announced that it has received clearance from the U.S. Food & Drug Administration (“FDA”) to market INVU™, a prescription-initiated, protocol-driven remote monitoring platform that offers measurements of fetal and maternal heart rate via a wireless, self-administered INVU…
Read MoreEchoNous, Inc. Announces CE Mark Approval for Its Healthcare AI KOSMOS Platform
EchoNous is very pleased to announce that its KOSMOS platform has been approved for CE Markets thanks to the unbending effort of its engineering, operations and regulatory teams. The company’s engineering team has developed a medical tool that, according to physician feedback, significantly increases provider confidence in bedside diagnostics and clinical decision-making with AI-assistance. The CE…
Read MoreNuclein Speeds Commercialization of First Ever Hand-Held PCR Test to Aid in COVID-19 Pandemic
Nuclein LLC announces plans to expedite the commercialization of its Nuclein™ Hand-Held PCR Test. The company’s disposable, all-in-one, self-test device for infectious disease diagnosis does not require technical expertise and provides battery-powered, sample-to-answer results in under one hour, without the need to mail in a sample. The company anticipates moving into product manufacturing soon, with the…
Read MoreIntuitive Surgical Among 1st Big Medtech Companies to Warn of Costly Pandemic Disruptions
Intuitive said the disruptions it’s felt to date in China, Korea, Taiwan and Italy “do not represent a material portion of our current procedure volume.” n Intuitive’s most critical U.S. market, infections are rising and rapid implementation of mitigation strategies more tangibly threatens the company’s goals for the year. During its most recent earnings call…
Read MoreHologic’s Molecular Test for the Novel Coronavirus Receives FDA Emergency Use Authorization
Hologic, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for the Company’s new Panther Fusion® SARS-CoV-2 assay, a molecular diagnostic test that detects SARS-CoV-2, the virus that causes COVID-19 disease. Hospital, public health and reference laboratories can perform the test on Hologic’s Panther Fusion system, a fully automated,…
Read More