Diagnostics & Healthcare News
Boston Scientific to acquire Augmenix for up to $600M
Boston Scientific said yesterday that it put $600 million on the table for Augmenix and its SpaceOar device. The deal for Bedford, Mass.-based Augmenix calls for $500 million in up-front cash and another $100 million pegged to sales-based milestones, Boston said. The SpaceOar hydrogel device, which won CE Mark approval in the European Union in 2010 and 510(k) clearance from…
Read MoreFDA grants Breakthrough Device status for Dthera’s Alzheimer’s Treatment
Dthera™ Sciences, the leading digital therapeutic company focusing on the elderly and individuals with neurodegenerative diseases, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to the Company’s development-stage product, DTHR-ALZ. DTHR-ALZ, if granted approval, would become the first non-pharmacological prescription treatment for the symptoms of Alzheimer’s disease. The…
Read MoreExact Sciences Signs Partnership with Pfizer to Market Cologuard Tests
Sales representatives of Pfizer Inc., the world’s largest pharmaceutical company, will join Exact Sciences Corp.’s sales representatives in selling the company’s non-invasive screening test for colorectal cancer to physicians and health systems under an agreement announced Wednesday. Pfizer will make at least 625,000 sales calls a year for Exact Sciences under the agreement. The two…
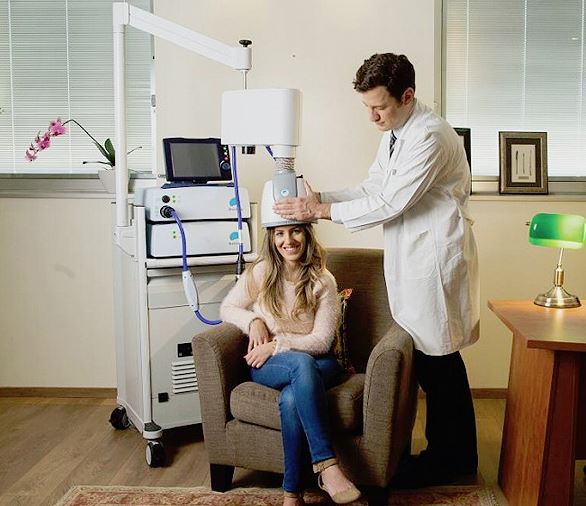
Read MoreFDA Clears Brainsway TMS System to Treat OCD
On Friday, the FDA said it granted de novo approval for Brainsway‘s deep transcranial magnetic stimulation system, now indicated for treating obsessive compulsive disorder. The TMS system uses magnetic fields to simulate nerves in the brain, and has been shown to reduce the severity of OCD in patients. A 100-patient randomized, multi-center study of the device…
Read MoreFirst Non-Radioactive Device for Breast Cancer Receives FDA Approval
Endomag, the surgical guidance company, announced that it has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for Magtrace, the first non-radioactive, dual-tracer for lymphatic mapping in patients with breast cancer undergoing a mastectomy. Magtrace is a liquid marker designed to follow the route that cancer cells are most likely to…
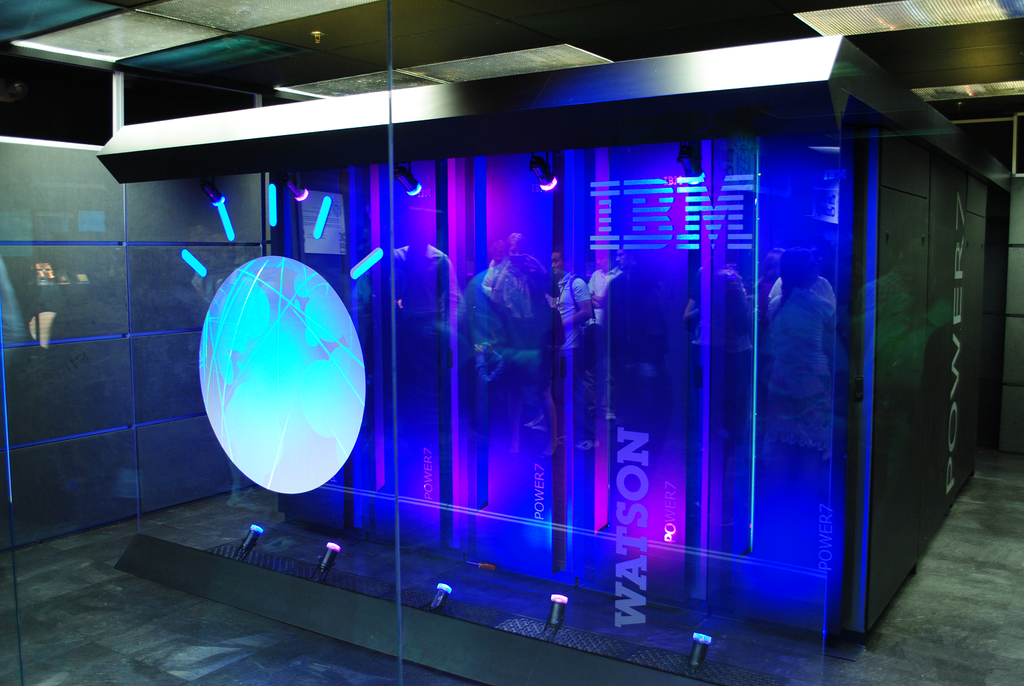
Read MoreIBM’s Watson Recommended “Unsafe and Incorrect” Cancer Treatments
Last year, studies presented at the American Society of Clinical Oncology’s annual meeting showed that IBM Watson was pretty darn good at creating treatment plans for cancer patients. Turns out, however, that the AI is still far from perfect: according to internal documents reviewed by health-oriented news publication Stat, some medical experts working with IBM on its Watson…
Read MoreHouse Votes to Repeal Obamacare’s Medical Device Tax
The U.S. House of Representatives voted 283-132 this week to repeal the 2.3% tax levied upon medical devices as part of the Affordable Care Act. The medical device tax, which has long been decried by the industry as a measure that squashes innovation and kills jobs, has been on hold since 2014. The tax’s moratorium…
Read MoreBiotech Manufacturing is Booming with Growth of Biologics and Gene Therapies
Manufacturing is the non-glamorous side of biopharma, although its impact on the economy shouldn’t be underestimated. For example, a recent report published by TEConomy and BIOtitled, “Investment, Innovation and Job Creation in a Growing U.S. Bioscience Industry 2018,” reported that the average wage in bioscience manufacturing was $64,860. It also reported that the six largest employer states in the drugs…
Read MoreAxonics Receives CE Mark for Sacral Neuromodulation External Trial System
Axonics Modulation Technologies, Inc., developer of the first rechargeable Sacral Neuromodulation (r-SNM™) system for the treatment of urinary and bowel dysfunction, announced today that it has received the CE mark for its Sacral Neuromodulation External Trial System. Axonics received CE mark in June 2016 for its miniaturized implantable neurostimulator, tined lead, programmers, charger and related accessories. The External Trial System…
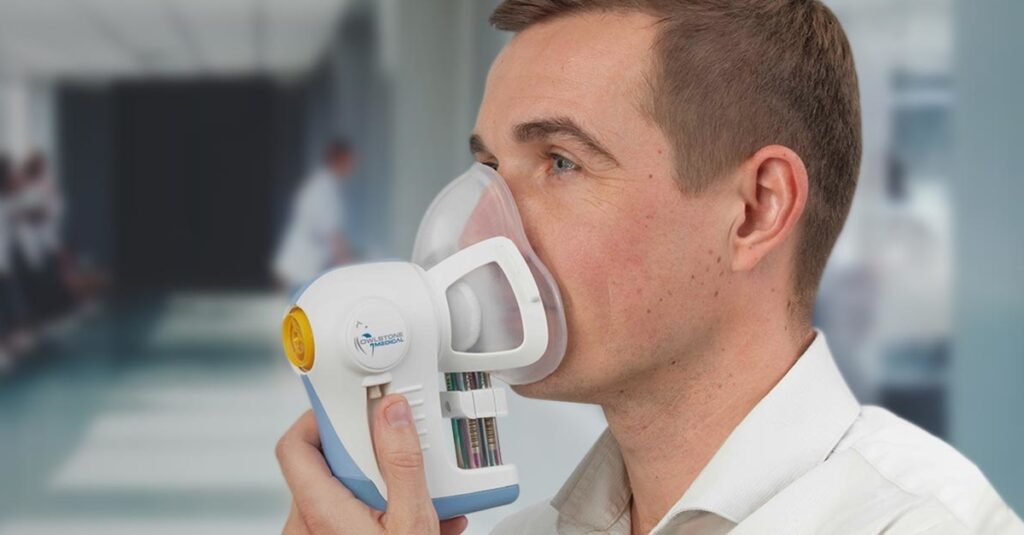
Read MoreBreath Biopsy Platform Wins UK’s Most Prestigious Award for Innovative Engineering
Owlstone Medical, a diagnostics company developing a breathalyzer for disease, has announced the team behind the Company’s unique Breath Biopsy® platform has been named the winner of the MacRobert Award 2018 by the Royal Academy of Engineering. Owlstone Medical’s Breath Biopsy platform, including the ReCIVA Breath Sampler, has opened up the potential for earlier diagnosis…
Read More