Orthopedics and Spine
Stryker Will Divest Total Ankle Business to get FTC Blessing for Wright Medical Acquisition
The Federal Trade Commission will require medical device companies Stryker Corp. and Wright Medical Group N.V. to divest all assets related to Stryker’s total ankle replacements and finger joint implant products to remedy concerns that Stryker’s proposed $4 billion acquisition of Wright will harm competition in those markets. Under the terms of the consent agreement, the companies…
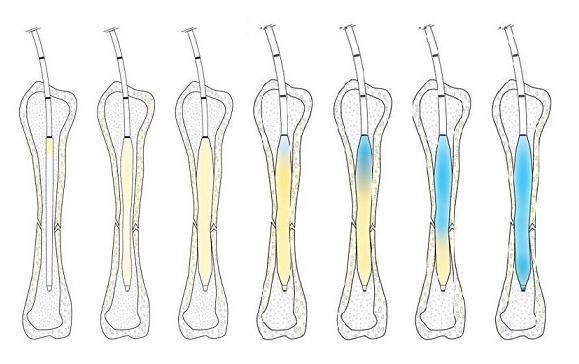
Read MoreIlluminOss Medical Receives FDA Clearance for Use in Femur and Tibia Fractures as a Supplement to Approved Hardware
IlluminOss Medical, a medical device company focused on minimally invasive orthopedic fracture repair, today announced an expanded U.S. Food and Drug Administration (FDA 510k) clearance for its Photodynamic Bone Stabilization System. The new clearance allows IlluminOss to be used in femur and tibia fractures as supplemental fixation to FDA-cleared fracture fixation systems. “Femur and tibia fractures can…
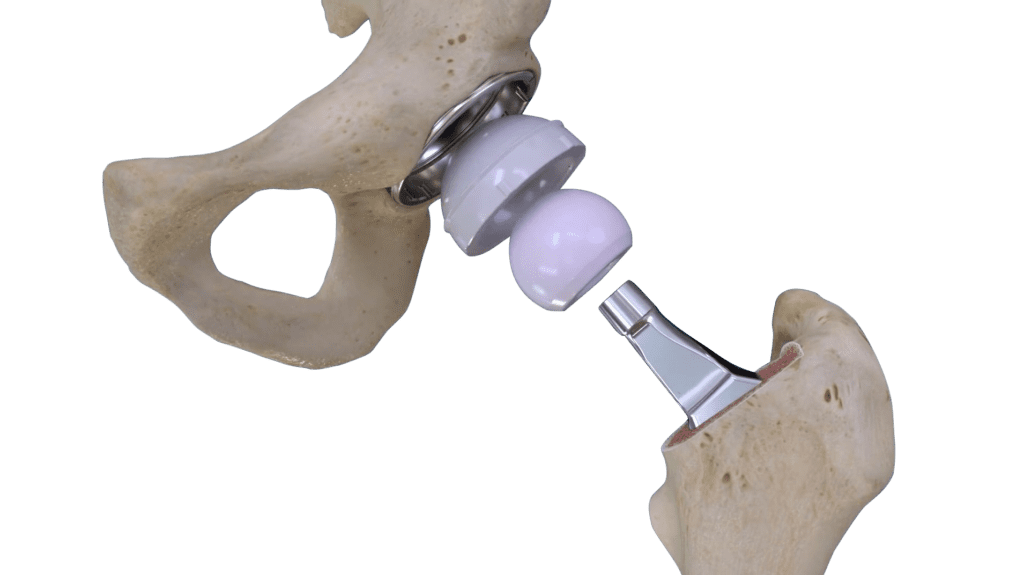
Read MoreConformis Announces 510(k) Clearance for Cordera Hip System
Conformis, Inc. today announced 510(k) clearance by the U.S. Food and Drug Administration of the Company’s new Cordera Hip System. The first product in the Conformis hip product line, the Conformis Hip System, was launched commercially in November 2019 and provided a patient-specific stem. The Cordera Hip System is an uncemented, primary total hip replacement…
Read MoreCartiHeal Receives FDA “Breakthrough Device Designation” for the novel Agili-C Implant
CartiHeal, developer of Agili-C, a proprietary implant for the treatment of cartilage lesions in arthritic and non-arthritic joints, announced today that FDA has granted Breakthrough Device Designation for the Agili-C implant. FDA’s Breakthrough Device Program is reserved for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases…
Read MoreSimplify Medical Receives FDA Approval for 1-Level Simplify Disc PMA
Simplify Medical, a privately-held company focused on cervical spinal disc arthroplasty and developer of the Simplify® cervical artificial disc, today announced U.S. Food and Drug Administration (FDA) Approval for the Simplify Disc Pre-Market Application (PMA) for 1-level indications. Simplify Disc achieved superiority to the fusion control on the composite primary endpoint. David Hovda, President and CEO…
Read MoreSmith+Nephew Acquires Integra Lifescience’s Extremity Orthopedics Business for $240
Smith+Nephew, the global medical technology business, announces that it has agreed to acquire the Extremity Orthopaedics business of Integra LifeSciences Holdings Corporation for $240 million. The acquisition supports Smith+Nephew’s strategy to invest in higher-growth segments. This acquisition will significantly strengthen Smith+Nephew’s extremities business by adding a combination of a focused sales channel, complementary shoulder replacement and upper…
Read MoreTHINK Surgical and Total Joint Orthopedics Announce Collaboration on Robotic Knee Surgery
Total Joint Orthopedics (TJO) announced today a new collaboration with THINK Surgical®, Inc., an advanced orthopedic robot technology company based in Fremont, CA. Under the terms of the agreement, THINK Surgical will develop software allowing TJO’s Klassic® Knee System to be implanted using THINK’s TSolution One® Total Knee Application and both companies will co-market the solution upon…
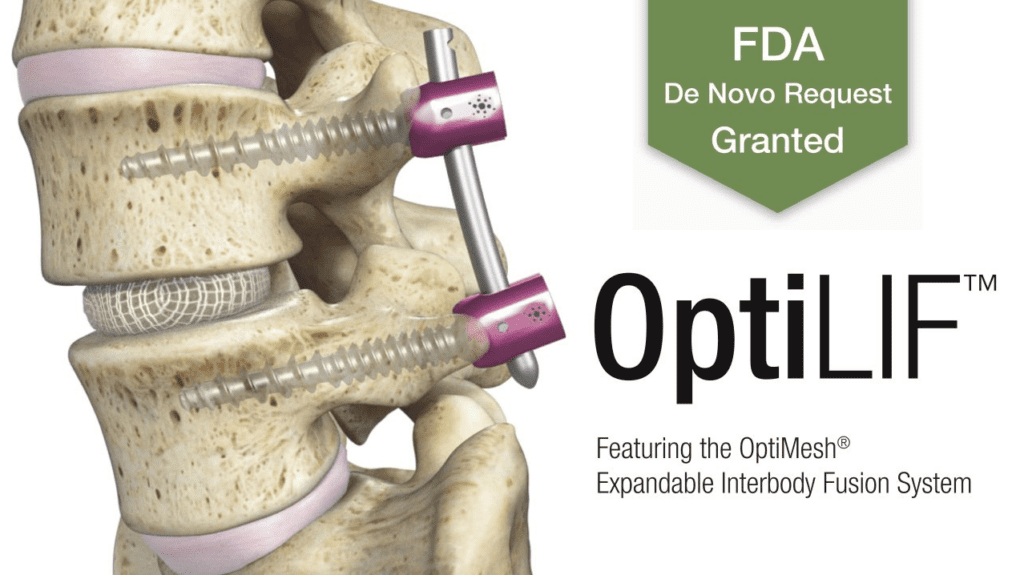
Read MoreSpineology Gets FDA De Novo Grant of Minimally Invasive OptiMesh Expandable Interbody Fusion System
Spineology Inc., an innovator in anatomy-conserving surgery, is excited to announce the FDA grant of its proprietary Spineology Interbody Fusion System, now called the OptiMesh Expandable Interbody Fusion System. The grant follows the successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption (IDE) trial. OptiMesh is a unique mesh device that expands…
Read MoreCerapedics Announces Canadian Approval of its Next-generation Bone Graft
Cerapedics, a private ortho-biologics company, today announced the Health Canada approval of the company’s next-generation bone graft called i-FACTOR+ Matrix, making it the first market to approve the commercial launch of this product. “We are excited to announce Canadian regulatory approval of our next-generation product, based on P15 osteogenic cell-binding peptide , i-FACTOR+ Matrix,” said…
Read MoreFDA Clears SurGenTec Synthetic Bone Graft
SurGenTec, a privately held spine and orthopedic technology company, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its proprietary OsteoFlo NanoPutty- Quadphasic Synthetic Bone Graft. OsteoFlo NanoPutty is a novel bone graft solution featuring the world’s first, and only quadphasic synthetic bone graft particles with nano-surface…
Read More