Cardiovascular / Cardiology
Bardy Diagnostics Announces Growth Capital Funding
CHARLOTTE, N.C., Jan. 5, 2017 /PRNewswire/ — Bardy Diagnostics, Inc. (“BardyDx” or “the Company”), which has developed and is commercializing the Carnation Ambulatory Monitor (“CAM™”), the world’s smallest and lightest P-wave centric™ ambulatory cardiac monitor and arrhythmia detection device, today announced that it has raised a significant round of growth capital from experienced healthcare investors SV…
Read MoreAbbott Labs Plans to Wrap Up St. Jude Medical Deal Jan. 4
Abbott Laboratories intends to close its deal to buy medical device maker St. Jude Medical just after the new year, now that it has received permission from U.S. and international antitrust regulators scrutinizing the $25 billion acquisition. Abbott Labs, in suburban Chicago, said Friday it intends to wrap up the deal on Jan. 4; originally,…
Read MoreNew Cardiac Tool Helps Repair Holes in Children’s Hearts
It’s always important to check your work. A new cardiac imaging tool allows surgeons to repair serious residual holes in the heart that may occur when repairing a child’s heart defect. The transesophageal echocardiography (TEE) allows surgeons to identify intramural ventricular skeptical defects—or holes in the wall between two heart chambers. “These defects, which can…
Read MoreJ&J’s Codman Neuro Picks Up Pulsar Vascular
Johnson & Johnson‘s (NYSE:JNJ) Codman Neuro said last week it picked up Pulsar Vascular, which produces the PulseRider device for treating brain aneurysms, for an undisclosed amount. The PulseRider is designed to shore up the blood vessels around wide-necked aneurysms at or near a bifurcation of the basilar tip or carotid terminus and facilitate the implantation of…
Read MoreHarvard Creates World’s First 3D Printed Heart-on-a-chip
3D Printed technology have awed our society in so many ways. They have created multiple advances that span from construction to food, but that’s not all that they’re capable of. Researchers at Harvard University have developed the first entirely 3D-printed organ-on-a-chip with integrated sensing. This new fabrication method can very much change how data is…
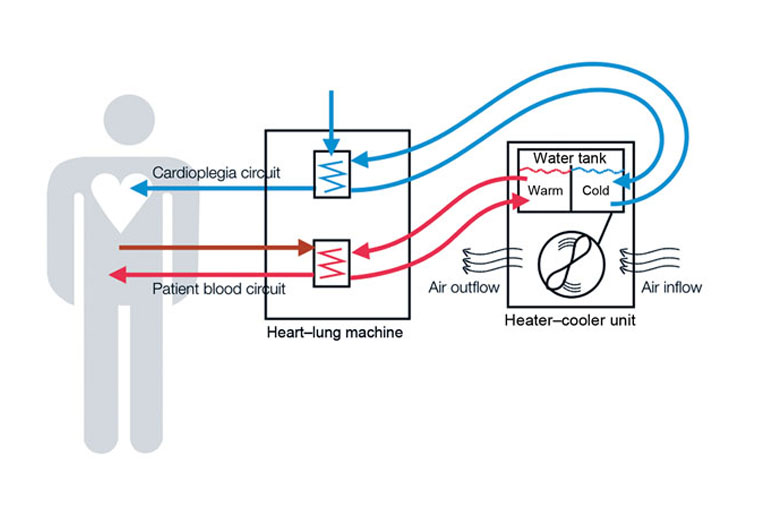
Read MoreDeadly Infections Linked To Heart Surgery Device Highlight Holes In FDA Monitoring
At first, Vincent Karst, 55, was recovering well from his open-heart surgery in March 2015. He resumed the activities he enjoyed, such as visiting car shows and eating out. But some months later, his condition mysteriously deteriorated. By fall he was so short of breath, nauseated and overwhelmed by fatigue that he needed to be rehospitalized in York, Pa. There, doctors diagnosed a new problem: a serious mycobacterial infection that…
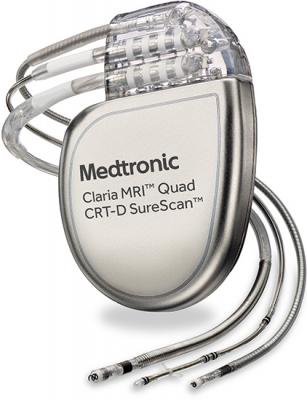
Read MoreMedtronic Receives FDA Approval for CRT Defibrillator That Improves Pacing
November 15, 2016 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the Claria MRI Quad Cardiac Resynchronization Therapy Defibrillator (CRT-D) SureScan device for patients with heart failure. The Claria MRI CRT-D is approved for scanning in both 1.5 and 3 Tesla (T) magnetic resonance imaging (MRI) machines, and features EffectivCRT,…
Read MoreAngioDynamics Secures $100M Loan & $150M Credit Line to Expand Market Share
AngioDynamics has secured a $100 million loan and a $150 million credit line as the medical device maker refinances debt, frees up cash and searches for opportunities to expand its market share. Chief financial officer Michael Greiner said the move will give the Latham, New York catheter and port maker flexibility to pursue new opportunities…
Read MoreHeart Transplant Recipient Receives the World’s First 31 Fr ProtekDuo™ Cannula in a Life-Saving TandemLung® Case
PITTSBURGH–(BUSINESS WIRE)–CardiacAssist, Inc. dba TandemLife announced today the commercial launch and first human use of their newest ProtekDuo dual lumen cannula at Cedars Sinai Heart Institute in Los Angeles. The cardiothoracic transplant team said the TandemLung and 31 Fr ProtekDuo were used in combination with the TandemHeart® pump to support a heart transplant recipient who…
Read MoreHeart Test Labs Appoints Industry Veteran Brian Allen as it Prepares for Commercial Device Launch
WESTLAKE, Texas–(BUSINESS WIRE)–Heart Test Laboratories Inc., announced today the appointment of Brian L. Allen as Senior Vice President of Business Development. In this role, Mr. Allen will lead the company’s commercial launch of its MyoVista electrocardiographic device. He will be responsible for building the sales team and distribution partners on a global basis. Mr. Allen…
Read More