Cardiovascular / Cardiology
Philips to Acquire Intact Vascular for $275M
Royal Philips, a global leader in health technology, today announced that it has signed an agreement to acquire Intact Vascular, Inc., a U.S.-based developer of medical devices for minimally-invasive peripheral vascular procedures. Intact Vascular will enhance Philips’ image-guided therapy portfolio, combining Philips’ interventional imaging platform and diagnostic and therapeutic devices with Intact Vascular’s unique, specialized…
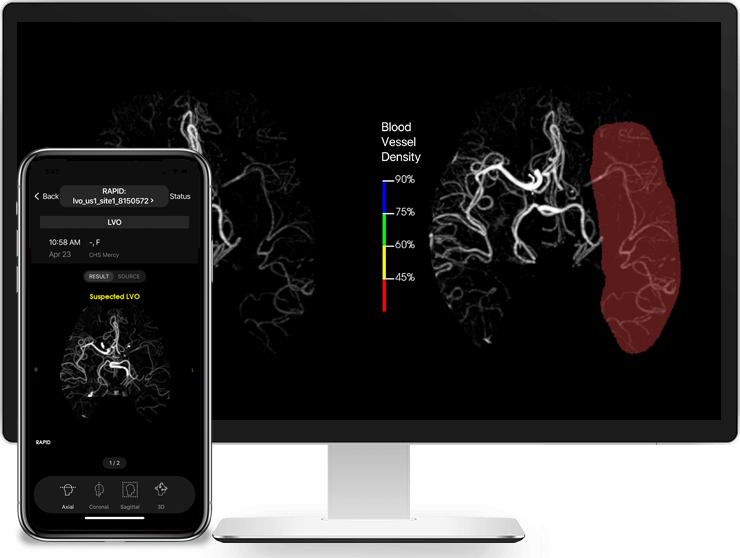
Read MoreRapidAI Receives FDA Clearance of Rapid LVO For Identification of Suspected Large Vessel Occlusions
RapidAI, the worldwide leader in advanced imaging for stroke, today announced that Rapid LVO has received Food and Drug Administration (FDA) clearance for detecting suspected LVOs (Large Vessel Occlusions). Rapid LVO helps physicians speed up triage or transfer decision-making. Working in as few as 3 minutes, Rapid LVO uses a vessel tracker in conjunction with…
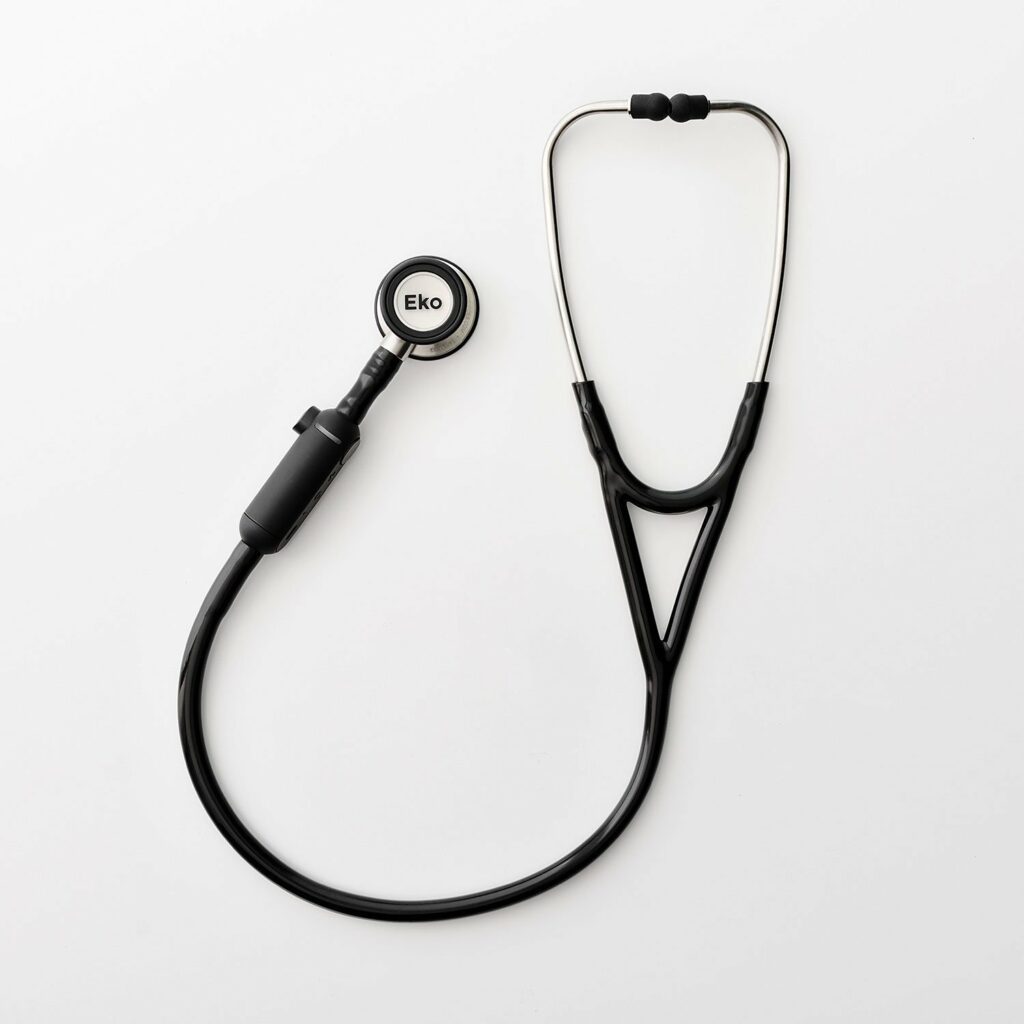
Read MoreEko and AstraZeneca Announce Digital Cardiovascular Health Partnership
Eko today announced a global collaboration with AstraZeneca to accelerate the development of digital health tools for the earlier screening of cardiovascular diseases, including heart failure. “There are millions of people living with or at risk for heart failure. AstraZeneca’s collaboration with Eko will tap into cutting-edge digital health technologies that could allow us to…
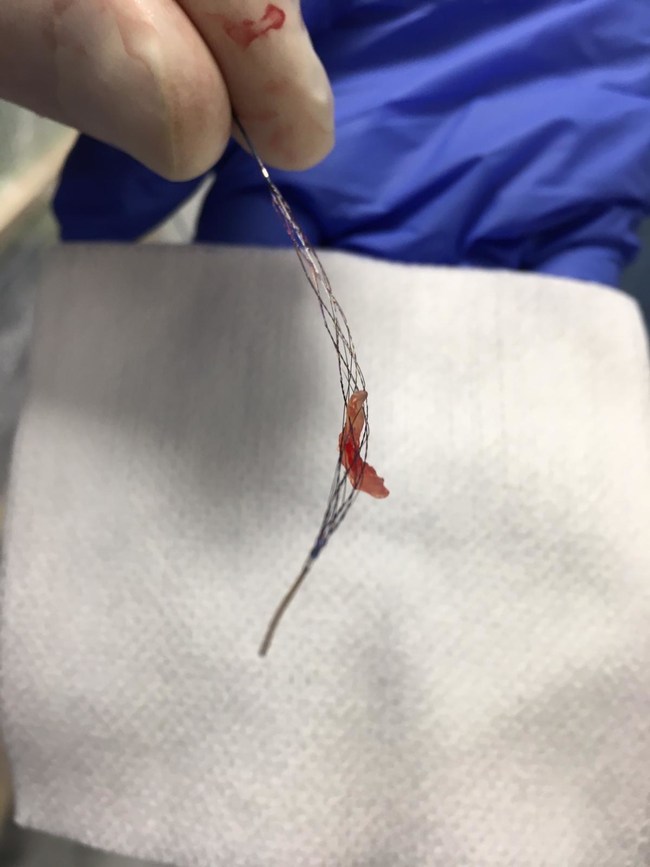
Read MoreRapid Medical Receives CE Mark for Stentriever
Rapid Medical, a company focused on the development of next generation neurovascular devices, has announced that it received CE Mark for TIGERTRIEVER XL. In addition, the first patients have been treated successfully with the device. The TIGERTRIEVER family of stentrievers are the first-ever adjustable, fully visible clot retrievers designed to treat ischemic stroke. Thousands of…
Read MoreArtio Medical Acquires Flow Forward Medical, Expanding Peripheral Vascular Portfolio
Artio Medical, Inc. (Artio) today announced it has acquired Flow Forward Medical, Inc. (Flow Forward), a medical device company developing innovative methods for establishing and maintaining high-quality vascular access sites to improve outcomes for hemodialysis patients. This stock-for-stock merger transaction in which Flow Forward merged with and into Artio was approved by the Board of…
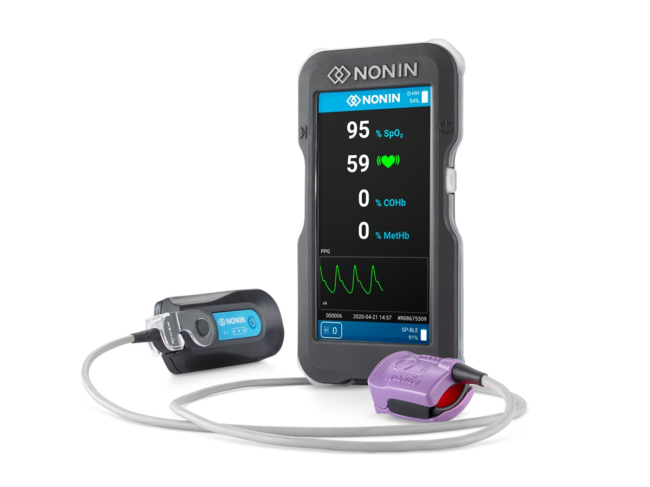
Read MoreFDA Clears Nonin Medical’s Flagship Product
Plymouth, Minn.-based Nonin Medical Inc. gained a U.S. FDA 510(k) clearance for its Co-Pilot wireless hand-held multiparameter system (H500). The system is expected to be used by first responders to evaluate various oxygenation and respiratory-related parameters in patients after incidents such as cardiac arrest, traumatic injury, carbon monoxide or smoke inhalation. It’s also expected to prove useful…
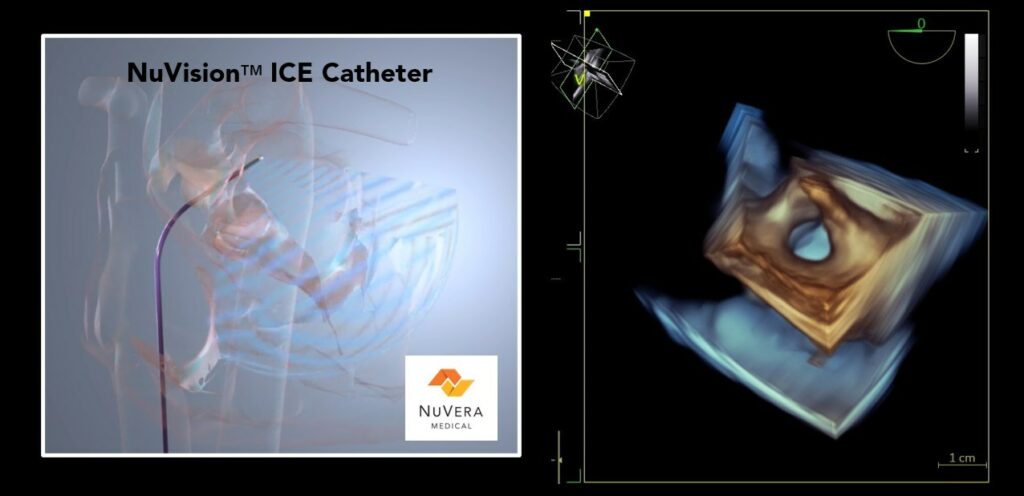
Read MoreNuVera Medical Announces Successful First-in-Human Use of NuVision ICE Catheter
NuVera Medical Inc. (NuVera), a portfolio company of Shifamed LLC, focused on enabling a new era in transcatheter cardiac interventions through the advancement of real-time 3D intracardiac echocardiography (4D ICE), announced today initiation of the company’s first-in-human clinical trial to evaluate the performance of its novel NuVision™ ICE Catheter. The first study participant was successfully treated this…
Read MoreCVRx Announces Publication of BeAT-HF Clinical Study Results in the Journal of The American College of Cardiology
CVRx, Inc., a private medical device company, announced today that its BeAT-HF phase III randomized clinical trial results were published in the Journal of the American College of Cardiology (“JACC”). Results from the trial were used to obtain Premarket Approval (PMA) from the United States Food and Drug Administration (“FDA”) to market its BAROSTIM NEO…
Read MoreLeMaitre Vascular Buys Artegraft for $90M
LeMaitre Vascular, Inc. announced that it has acquired the business and assets of Artegraft, Inc. for $90.0 million, including $72.5 million in cash paid at closing ($65.0 million to Artegraft plus $7.5 in escrow to be released December 31, 2021) as well as potential earnout payments of $17.5 million payable based upon future sales of…
Read MoreBreakthrough Device Designation Given for preCARDIA’s Catheter Based Heart Failure Treatment
preCARDIA, Inc., has announced that the company’s catheter based system for treating volume overload in patients with acutely decompensated heart failure (ADHF) has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA). The FDA’s Breakthrough Device Program was established for medical technologies that have the potential to provide more…
Read More