Cardiovascular / Cardiology
First US Patients Treated Using the CardioFocus HeartLight X3 Ablation System
CardioFocus, Inc. today announced that the first U.S. patients have been treated commercially with the recently-approved HeartLight® X3 Endoscopic Ablation System. The revolutionary cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AFib), the most common heart rhythm disorder. Henry D. Huang, M.D., FACC, FHRS of Rush University Medical Center in Chicago, and David Kenigsberg M.D., FACC, FHRS…
Read MoreNeovasc Closes $11.5 Million Offering
Neovasc Inc. (“Neovasc” or the “Company”), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, and minimally invasive devices for the treatment of refractory angina, announced today that it has closed its previously announced registered direct offering (the “Offering”) priced at-the-market under Nasdaq rules of an aggregate of 3,883,036 units (the…
Read MoreShockwave Medical Announces That CMS Has Created New Codes for Intravascular Lithotripsy
Shockwave Medical, Inc., a pioneer in the development and commercialization of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, announced today that the Centers for Medicare & Medicaid Services (CMS) has issued new codes for IVL procedures performed in peripheral arteries in both the hospital outpatient and inpatient settings. The new Healthcare Common Procedure…
Read MoreVasQ External Support Awarded Breakthrough Device Designation by the FDA
The FDA has designated Laminate Medical’s VasQ™ External Support for the creation of arteriovenous fistulas (AVF) in hemodialysis patients as a Breakthrough Device. The FDA Breakthrough Device Program is intended to provide patients and doctors timely access to medical devices that are more effective than the current standard of care for life-threatening or irreversibly debilitating…
Read MoreFDA Clears Innovative Angioplasty Scoring and Cutting Platform
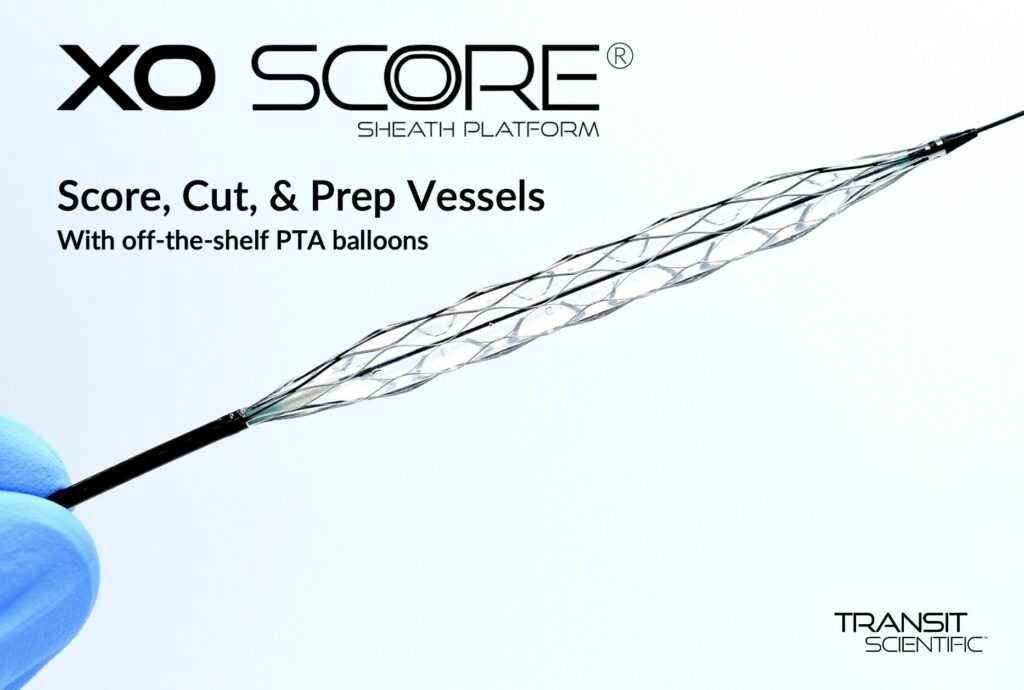
Transit Scientific announced the FDA cleared the XO Score® Percutaneous Transluminal Angioplasty (PTA) Scoring Sheath platform for use in iliac, ilio-femoral, popliteal, infra-popliteal, and renal arterial plus synthetic and/or native arteriovenous hemodialysis fistula. Angioplasty is performed with expandable polymer balloon catheters to dilate stenosed, or narrowed, vessels. Calcified, fibrous, and/or resilient stenosis may require special scoring…
Read MoreAesculap Inc. Announces U.S. Launch of M.blue Hydrocephalus Valve
Aesculap Inc., in partnership with The Christoph Miethke GmbH & Co. KG (MIETHKE), is pleased to announce the launch of the M.blue valve, the latest generation of Hydrocephalus valve technology. Its unique gravitational technology is integrated with a fixed differential pressure unit in one valve, allowing for a simple, position-dependent solution. The M.blue valve is…
Read MoreFDA Issues Emergency Use Authorization for Impella RP as Therapy for COVID-19 Patients with Right Heart Failure
The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Abiomed (NASDAQ: ABMD) manufactures Impella RP. Impella RP is a temporary heart pump that provides circulatory support for patients who develop right side ventricular failure.…
Read MoreNeovasc Has Filed for CE Mark for Tiara TA Transapical Mitral Valve Replacement System
Neovasc, Inc., a leader in the development of minimally invasive transcatheter mitral valve replacement technologies, and minimally invasive devices for the treatment of refractory angina, today announced that the Company has filed for CE Mark for its Tiara TA Transapical mitral valve replacement system. Mitral Valve disease is one of the most common forms of…
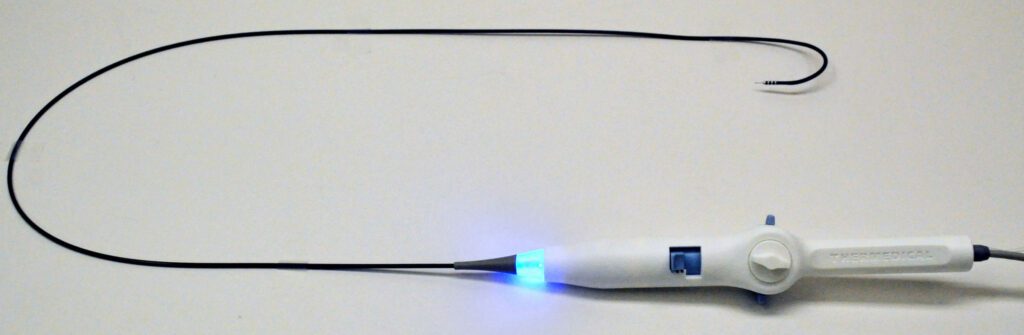
Read MoreThermedical’s Groundbreaking SERF Ablation System Earns FDA’s Breakthrough Device Designation
Thermedical®, a developer of thermal-ablation medical systems to treat ventricular tachycardia (VT), today announced that it has received Breakthrough Device Designation from the U.S. Food & Drug Administration (FDA) for its Saline Enhanced Radiofrequency (SERF) Ablation system and Durablate® catheter. The FDA Breakthrough Devices Program is intended to help patients receive more timely access to technologies that…
Read MoreFDA Issues Emergency Use Authorization for Eko’s ECG-based Low Ejection Fraction Screening Algorithm, Designed to Improve Detection of Heart Failure During COVID-19 Pandemic
Eko, a digital health company building AI-powered screening and telehealth solutions to fight cardiovascular disease, today announced that the U.S. Food and Drug Administration (FDA) has issued the company an Emergency Use Authorization (EUA) for its novel ECG-based algorithm that can provide an easily accessible, rapid screening test for low ejection fraction (low EF), a weak heart…
Read More