Cardiovascular / Cardiology
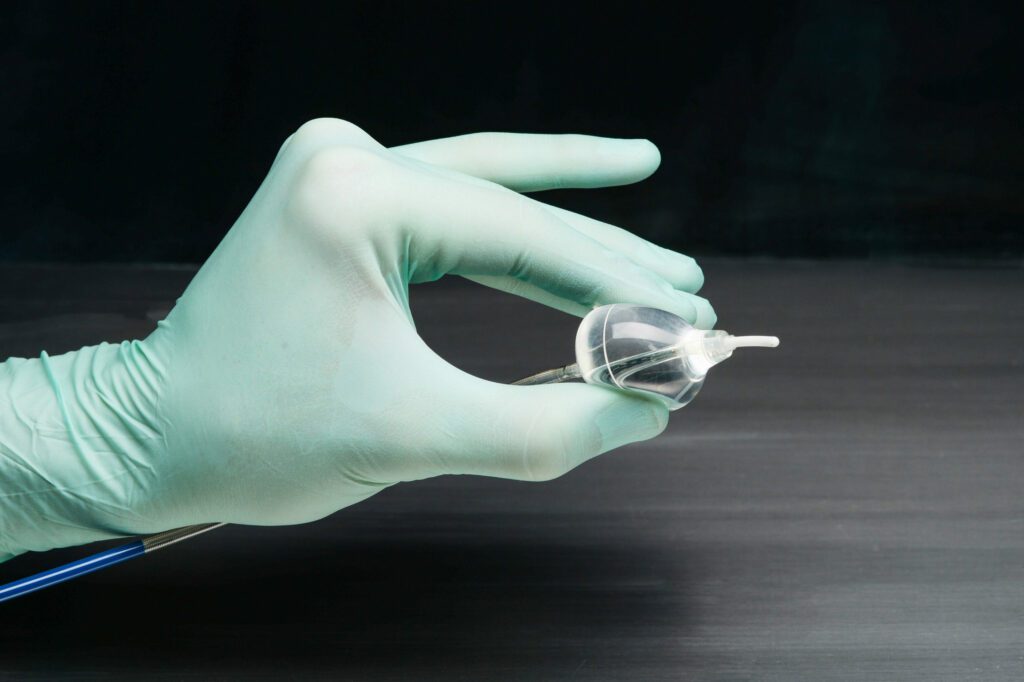
CardioFocus Announces US FDA Approval of HeartLight X3 System for the Treatment of Atrial Fibrillation
CardioFocus, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved the next-generation HeartLight® X3 Endoscopic Ablation System for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF). Approval of the HeartLight X3 System came as a result of a comprehensive submission, including outcomes from the study of 60 HeartLight X3 patients. In…
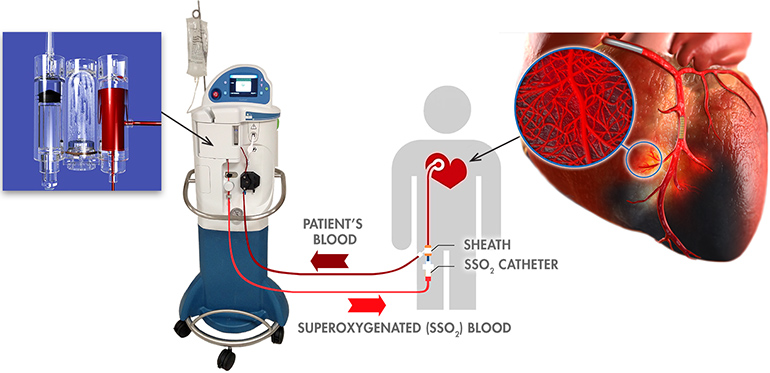
Read MoreZOLL TherOx Receives CE Mark Approval for Supersaturated Oxygen Therapy
ZOLL Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today it has received CE Mark approval to market and distribute its SuperSaturated Oxygen (SSO2) Therapy System in Europe. SSO2 Therapy provides interventional cardiologists with the first and only clinically proven treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage…
Read MoreFDA Grants Emergency Use Authorization to VitalConnect for Cardiac Monitoring in COVID-19 Patients
VitalConnect®, Inc., a leader in wearable biosensor technology, announced it was granted Emergency Use Authorization (EUA) status by the U.S. Food and Drug Administration (FDA) as part of the response to the COVID-19 pandemic. The FDA EUA will further enhance the capabilities of the VitalPatch and continuous patient monitoring technology, the Vista Solution. Under the FDA EUA, the…
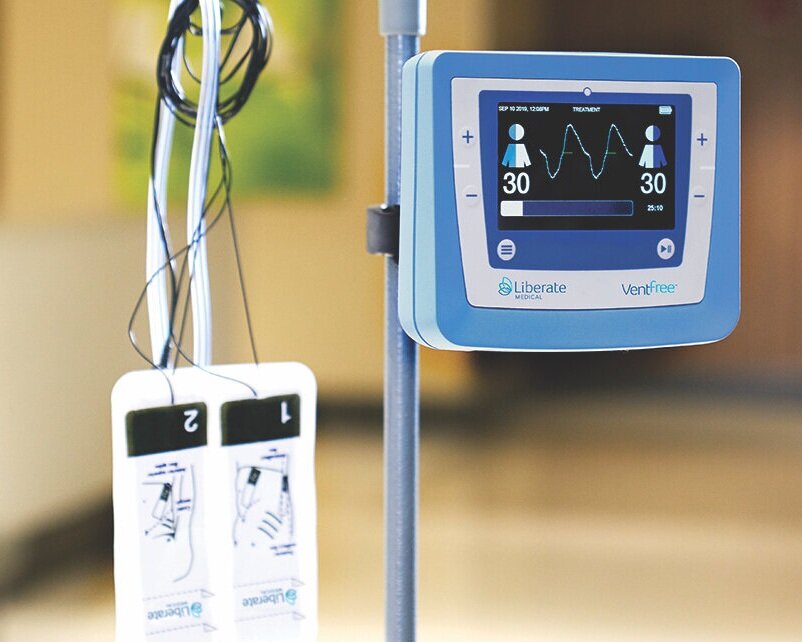
Read MoreVentFree Respiratory Muscle Stimulator Receives FDA Emergency Use Authorization for Use During COVID-19 Pandemic
Liberate Medical today announced that it has received Federal Drug Administration (FDA) Emergency Use Authorization for its VentFree™ Respiratory Muscle Stimulator, intended to be used to reduce disuse atrophy of the abdominal wall muscles, which may reduce the number of days adult patients require mechanical ventilation, including those patients with COVID-19. Reducing the time patients…
Read MoreAbiomed Expands Product Portfolio with Acquisition of Cardiopulmonary Support Technology to Improve Outcomes for Patients
Abiomed, maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation, including patients suffering from cardiogenic shock or respiratory failure such as due to ARDS,…
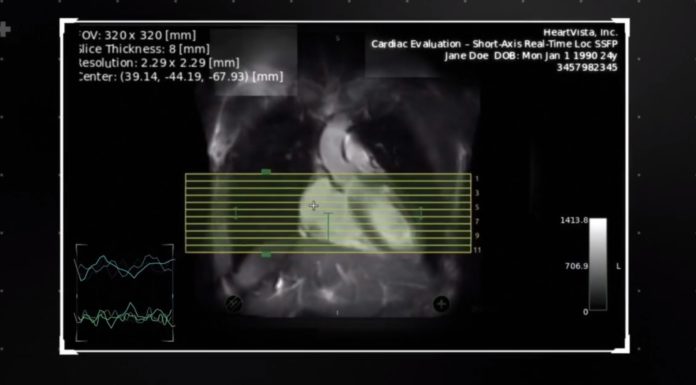
Read MoreHeartVista Closes $8.65M Series A Financing Led by Khosla Ventures
HeartVista, a pioneer in AI-assisted MRI solutions, today announced the closing of a $8.65M Series A financing round. The round was led by Khosla Ventures, Jeff Rothschild, Leslie Ventures, Open Field Capital, and additional investors. Combined with several grants from the National Institutes of Health (NIH), this latest raise brings the company’s total funding to…
Read MoreLungpacer Medical Receives FDA Emergency Use Authorization to Wean Patients Off Mechanical Ventilation During Covid Crisis
Lungpacer Medical announced today that the U.S. Food and Drug Administration has issued Emergency Use Authorization (EUA) for the Company’s novel Diaphragmatic Pacing Therapy System (DPTS) for immediate use in patients on invasive mechanical ventilators at high risk of weaning failure, including COVID-19 patients. “We are thrilled to hear about the FDA’s decision to grant…
Read MoreCagent Vascular Announces FDA 510(k) Clearance for Balloon Catheter
Cagent Vascular (Wayne, PA), a developer of serration technology for vessel dilatation in cardiovascular disease interventions, announces FDA 510(k) Clearance of its Serranator® PTA Serration Balloon Catheter for treating below-the-knee (BTK) lesions. The Serranator device is the first and only angioplasty balloon FDA Cleared and CE Marked that embeds serration technology into a semi-compliant balloon…
Read MoreFDA Grants ALung Emergency Use Authorization (EUA) to the Hemolung Respiratory Assist System (RAS) for the Treatment of COVID-19
ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced that the Food and Drug Administration (FDA) has granted the Company Emergency Use Authorization (EUA) designation to the Hemolung® Respiratory Assist System (RAS) for the treatment of Coronavirus Disease 2019 (COVID-19) patients. ALung…
Read MoreAliveCor and OMRON Announce Global Strategic Alliance for Comprehensive Remote Cardiovascular Monitoring
AliveCor, a leader in personal ECG products, and OMRON Healthcare, Co., Ltd., a global leader in personal heart health and wellness technology, today announced a global, strategic alliance that combines AliveCor’s ECG technology with industry-leading blood pressure devices from OMRON to better serve customers and expand access to remote patient care. This partnership aligns with global…
Read More