Surgery and Surgical Robotics
Intersect ENT Announces Agreement to Acquire Fiagon for $71M
Intersect ENT, Inc., a company transforming care for patients with ear, nose and throat (“ENT”) conditions, today announced it has entered into an agreement to acquire Fiagon AG Medical Technologies, a leader in electromagnetic surgical navigation solutions, for €60 million in cash. Under the terms of the transaction, Intersect ENT will make an initial €15 million payment at…
Read MoreMinnetronix Medical Wins FDA Clearance for Innovative Neurosurgical Access Platform
Minnetronix Medical, the company known for 25 years of developing and manufacturing products for medical device companies throughout the world, today announced that it has received FDA clearance for its first platform product: the MindsEye™ Expandable Port for neurosurgical procedures. This clearance represents an expansion of the company’s traditional offerings to include market-ready platforms. The…
Read MoreHologic Buys Accessa Health for $80M
Hologic, Inc., a global leader in women’s health, announced today that it has acquired Acessa Health Inc., a privately-held innovator in minimally invasive treatment for fibroids, for approximately $80 million in cash plus contingent payments based on future revenue growth. “Acquiring Acessa Health strengthens our leadership position in the GYN surgical space and broadens our…
Read MorePelvital Announces FDA Clearance of Flyte Pelvic Floor Device
Pelvital, a medical technology company focused on women’s health, announced it has received U.S. Food and Drug Administration (FDA) clearance for Flyte, a first-of-its-kind, non-invasive, intravaginal home-use device that is intended for strengthening of the pelvic floor muscles, which has been found to help women with stress urinary incontinence. According to the National Association for…
Read MoreU.S. FDA Grants Ethicon Breakthrough Device Designation for Monarch-enabled NeuWave Microwave Ablation Technology
Ethicon, part of the Johnson & Johnson Medical Devices Company, announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for transbronchial microwave ablation technology using robotic-assisted bronchoscopy, which is currently under development. The Breakthrough Devices Program is a voluntary program for certain medical devices that provide for more effective treatment or…
Read MoreAmbu Wins FDA Clearance for Single-use Duodenoscope Product
Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, announced today that the Ambu® aScope™ Duodeno has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). “At Ambu, we are determined to advance patient safety through innovative design of single-use devices, and we are excited to improve safety for the…
Read MoreFDA Approves Channel Medsystems’ Women’s Health Cryotherapy Device
Channel Medsystems, a company dedicated to bringing innovation to the delivery of women’s healthcare, today announced that the U.S. Food and Drug Administration (FDA) recently approved the newest Cerene Cryotherapy Device, a next-generation technology for the treatment of heavy menstrual bleeding in the office setting. Originally approved by the FDA in March 2019, Channel’s Cerene Device uses…
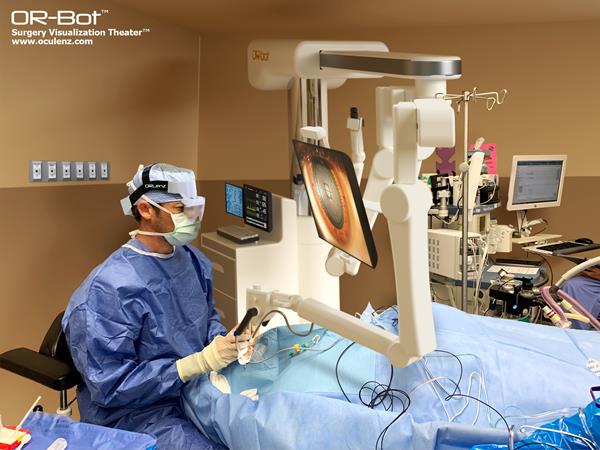
Read MoreOcutrx’s Oculenz Advances from AR Headset to Full Surgery Visualization Theatre
The maker of breakthrough augmented-extended reality (AR/XR) glasses that provide a radically new surgical viewing experience for retinal surgeons and patients alike, is launching new technology that provides the most modern options for surgery visualization and to remove “pain” for surgeons to improve surgeries, Ocutrx Vision Technologies, LLC announced today. The Ocutrx OR-Bot™️ Surgery Visualization Theatre™ will…
Read MoreCMS Approves SINUVA Sinus Implant for Reimbursement with New C-Code and Pass-Through Payment Status
Intersect ENT, Inc., a company dedicated to transforming care for patients with ear, nose and throat conditions, today announced that the Centers for Medicare and Medicaid Services (CMS) has approved SINUVA® (mometasone furoate) Sinus Implant for transitional pass-through payment status for reimbursement under the Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center Payment…
Read MoreTitan Medical Announces Development and License Agreements With Medtronic and Senior Secured Loan
Titan Medical Inc. (“Titan” or “Titan Medical”) (TSX: TMD) (Nasdaq: TMDI), a medical device company focused on the design and development of single-port robotic surgical technologies, announces that it has entered into a development and license agreement with Medtronic plc (“Medtronic”) (NYSE: MDT) to further the development of robotic assisted surgical technologies, as well as…
Read More