Surgery and Surgical Robotics
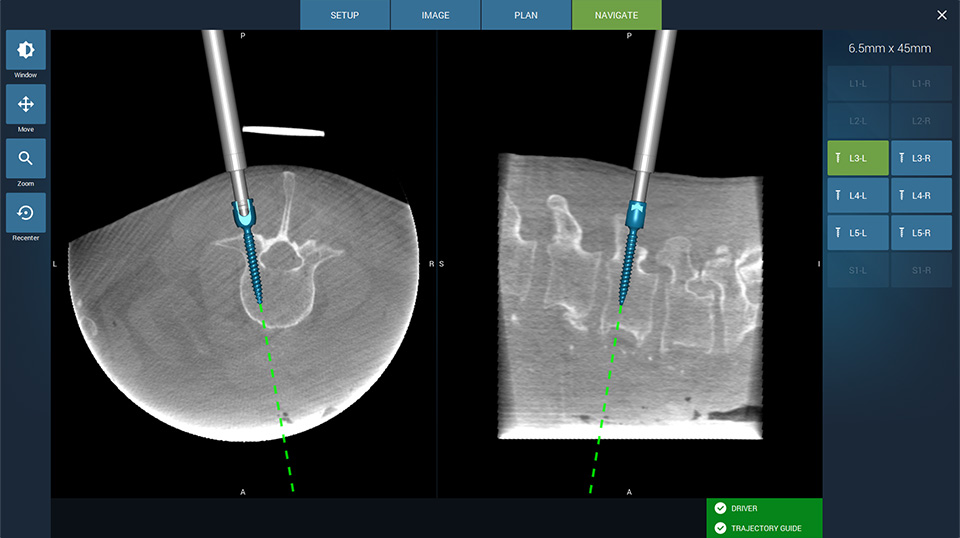
Fusion Robotics Wins FDA Clearance for 3D Imaging Robotic Targeting System
Fusion Robotics LLC, a spinal robotics and navigation company today announced receiving 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery in the U.S. market. The Fusion Robotics System addresses the key limitations of current spinal navigation and robotics systems by offering greater procedural efficiency with significantly less…
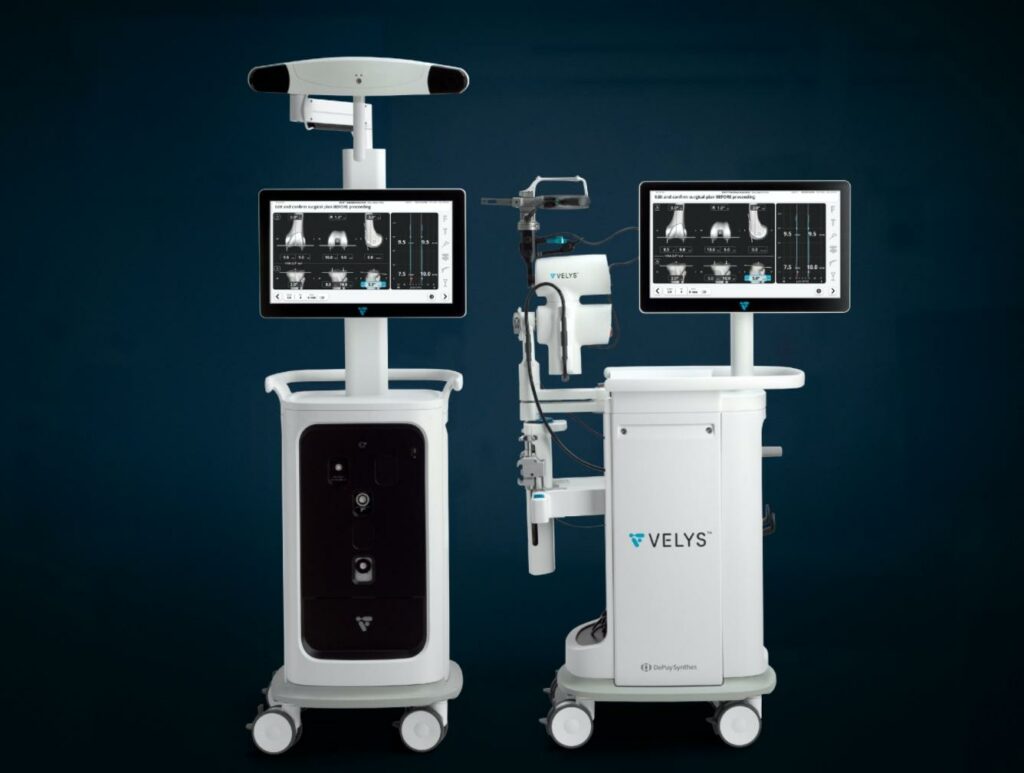
Read MoreDePuy Synthes Receives 510(k) FDA Clearance for VELYS Robotic-Assisted Solution Designed for Use with the ATTUNE Total Knee System
Today, The Johnson & Johnson Medical Devices Companies announced that DePuy Synthes has received 510(k) clearance from the U.S. Food and Drug Administration for the VELYS Robotic-Assisted Solution designed for use with the ATTUNE Total Knee System and its cleared indications for use. It will become part of the broader VELYS Digital Surgery Platform of…
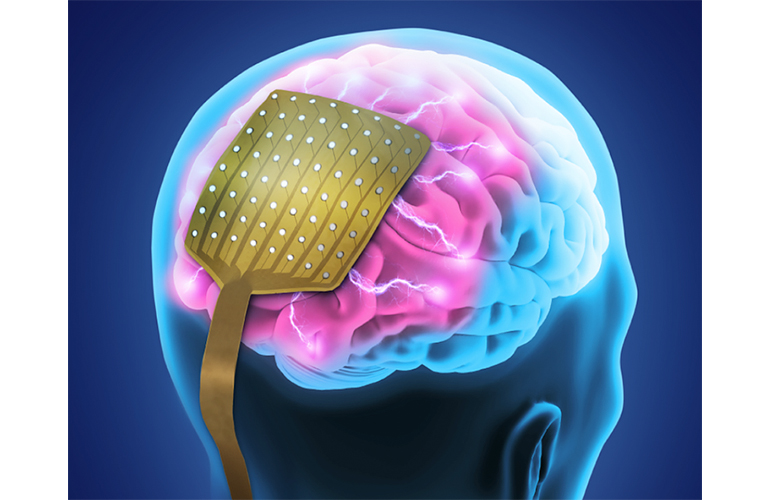
Read MoreNeuroOne Medical Announces First Human Commercial Use of its Evo Cortical Electrode at Mayo Clinic
NeuroOne Medical Technologies Corporation (OTCQB: NMTC; NeuroOne), a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, announced today the first human commercial use of its Evo Cortical Electrode at Mayo Clinic in Rochester, Minnesota. The procedure was performed by Jamie Van Gompel, M.D., from November 23rd through November 27th on a patient that had…
Read MoreZimmer Biomet Picks up Chest Surgery Toolmaker A&E Medical for $250M
Vance Street Capital LLC (“Vance Street”) is pleased to announce it has completed the sale of A&E Medical Corporation (“A&E Medical”) to Zimmer Biomet (NYSE: ZBH) (“Zimmer”), a global leader in musculoskeletal healthcare, for $250 million. The transaction marks the second exit out of Vance Street’s Sophomore Fund. Founded in 1968 and headquartered in Farmingdale,…
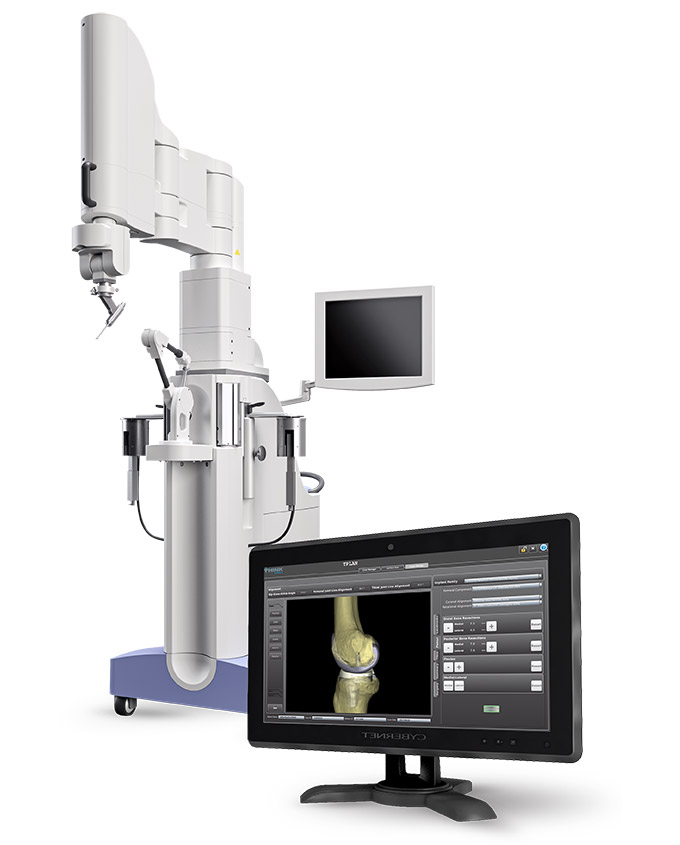
Read MoreTHINK Surgical Receives FDA Clearance of Second-Generation Active Robot
THINK Surgical, an innovator in the field of orthopedic active robot surgery, announced today that the Food and Drug Administration (FDA) has cleared the second-generation of the TSolution One Total Knee Application. The system features an active robot for total knee replacement, providing fully automated bone preparation, and gives surgeons a choice of implant options.…
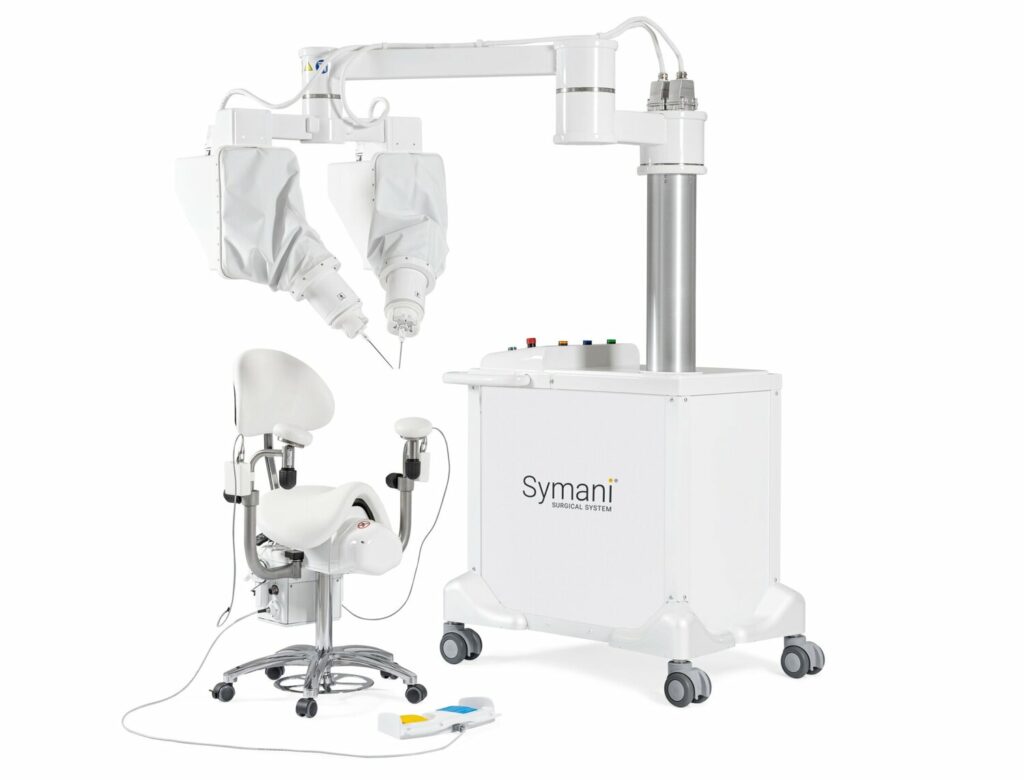
Read MoreMMI’s Symani Robotic Microsurgery System Cleared in Europe
MMI SpA, an Italian company dedicated to improving clinical outcomes for patients undergoing microsurgery, announced today the CE Mark, launch and first human use of its Symani® Surgical System in Europe for open microsurgical procedures. The first four robotic surgeries were successfully performed in Florence, Italy, including three complex, post-traumatic lower limb reconstructions as well…
Read MoreAllotrope Medical Announces FDA Clearance of StimSite
Allotrope Medical™ Inc., a company committed to advancing surgical safety and precision, today announced its FDA clearance of their device, StimSite. StimSite provides ob/gyns, general and colorectal surgeons the new ability to use their existing surgical instruments to help locate and identify ureters using electrical stimulation. Ureter identification is a critical step in safely advancing…
Read MoreMedical Microinstruments Announces CE Mark for Robotic Microsurgery with the World’s Smallest Wristed Surgical Instruments
MMI SpA, an Italian company dedicated to improving clinical outcomes for patients undergoing microsurgery, announced today the CE Mark, launch and first human use of its Symani® Surgical System in Europe for open microsurgical procedures. The first four robotic surgeries were successfully performed in Florence, Italy, including three complex, post-traumatic lower limb reconstructions as well as a post-oncological reconstruction…
Read MoreCrossBay Medical Receives FDA Clearance for CrossGlide ETS Plus
CrossBay Medical, Inc., a health technology company focused on improving the delivery of women’s healthcare, announced it has received clearance from the Food and Drug Administration (FDA) to market its CrossGlide ETS Plus. Similar to the CrossGlide ETS, Endometrial Tissue Sampler (which received FDA clearance this summer), the ETS Plus also enables medical providers to perform…
Read MoreSteris to acquire Key Surgical for $850 Million
STERIS plc (NYSE: STE) (“STERIS” or the “Company”) today announced that the Company has signed a definitive agreement to purchase Key Surgical, a portfolio company of Water Street Healthcare Partners, LLC, through a U.S. subsidiary for $850 million. STERIS anticipates that the acquisition will qualify for a tax benefit related to tax deductible goodwill. Adjusting for the present value of…
Read More