Archive for November 2019
Nevro’s Senza Omnia Spinal Cord Neurostimulation System Gains FDA Approval
Nevro Corp. (NYSE: NVRO), a global medical device company that provides innovative, evidence-based solutions for the treatment of chronic pain, today announced it has received approval from the U.S. Food and Drug Administration (FDA) for the Senza® Omnia™ Spinal Cord Stimulation (SCS) System. The Omnia system is the first and only SCS system designed to deliver Nevro’s…
Read MoreSonde Health Granted Foundational U.S. Patent for Use of Vocal Biomarkers in Health Assessments
Sonde Health, Inc., a leading company developing a voice-based technology platform to measure health when a person speaks, today announced that the United States Patent and Trademark Office issued U.S. Patent No. 10,475,530, a foundational patent that covers crucial aspects of Sonde’s vocal biomarker technology. The patent covers the use of Sonde’s technology to capture,…
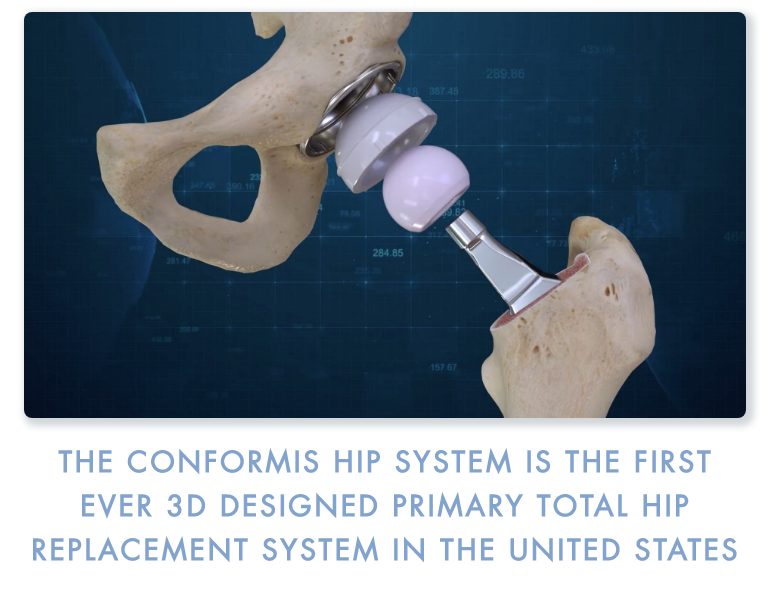
Read MoreConformis Announces FDA Clearance and Full Commercial Launch of Next Generation Hip System
Conformis, Inc. (NASDAQ:CFMS), a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture, and sell patient-specific arthroplasty joint replacement implants designed to fit each patient’s unique anatomy, today announced FDA clearance of the Company’s next generation Conformis Hip System. The Company will be featuring the new 3D-designed Conformis Hip System as…
Read MoreAirflow Sleep, a Sleep Solutions and Advocacy Company, Launches to Fight the Sleep Epidemic
Airflow Sleep, a total sleep solutions and advocacy company that empowers people to improve sleep quality and behavior, exited stealth mode today as it prepares to launch its ZO2 Sleep System for the consumer market. Each system contains a physician-designed, patented side or back sleeper pillow supporting all types of sleepers, an advanced sleep tracking…
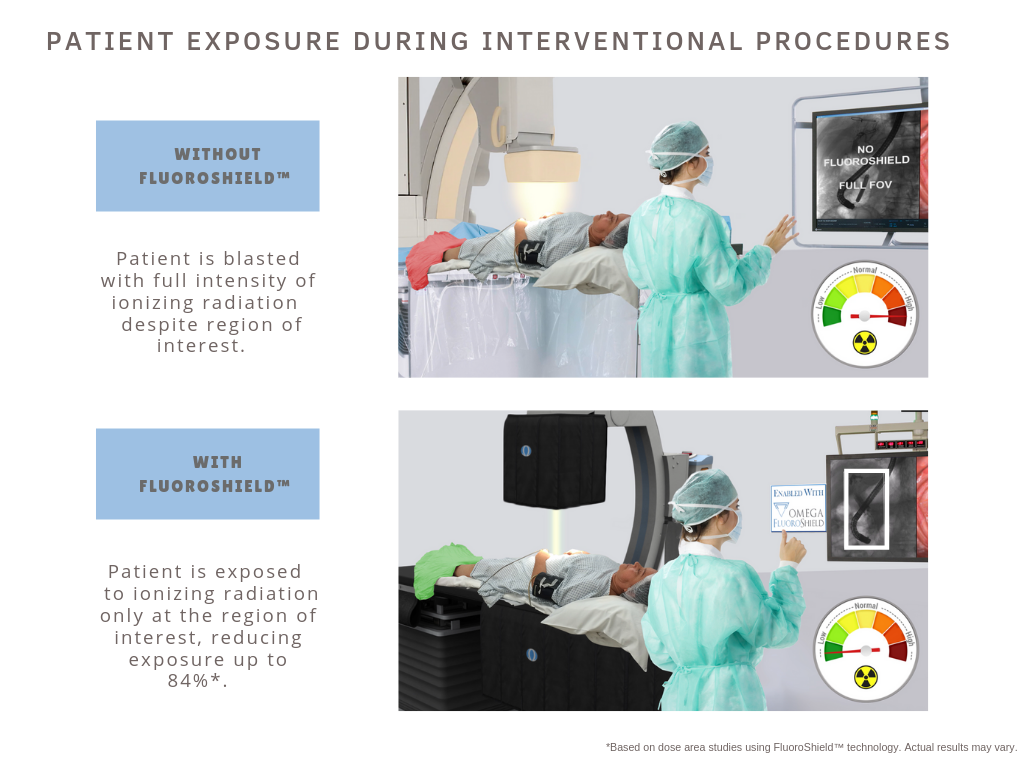
Read MoreOmega Medical Imaging First in the World to Receive FDA Clearance on Artificial Intelligence Imaging System that Reduces Radiation Exposure
Omega Medical Imaging, manufactures of Artificial Intelligence Fluoroscopy/Cine (AIF/C) Imaging systems, just announced the Food and Drug Administration 510 (k) clearance of FluoroShield™ with their 2020 Cardiac Flat Panel Detector. The unique FluoroShield™ system allows for auto collimation during interventional fluoro or cine cases while maintaining a perspective of surrounding anatomy. The blended image incorporates a lower…
Read MoreSaranas Announces First Commercial Case With Early Bird® Bleed Monitoring System in the U.S.
Saranas, Inc. announced completion of the first U.S. commercial case using the Early Bird® Bleed Monitoring System for real-time detection and monitoring of endovascular bleed complications. Dr. Robert Kipperman, co-director of the Structural Heart Disease Program at Morristown Medical Center, and Dr. Bledi Zaku, cardiothoracic surgeon, successfully used the Early Bird to monitor for bleed complications…
Read MoreEDAP to Introduce New Endo-UP Endourology Platform During the 2019 World Congress of Endourology
EDAP TMS SA, the global leader in therapeutic ultrasound, announced today the introduction of a new concept of Endourology Platform during the WCE congress, which is being held in Abu Dhabi, October 29 – November 2, 2019. Endo-UP® is a new conceptual product designed for the management of urinary stones. Responding to the rapidly evolving…
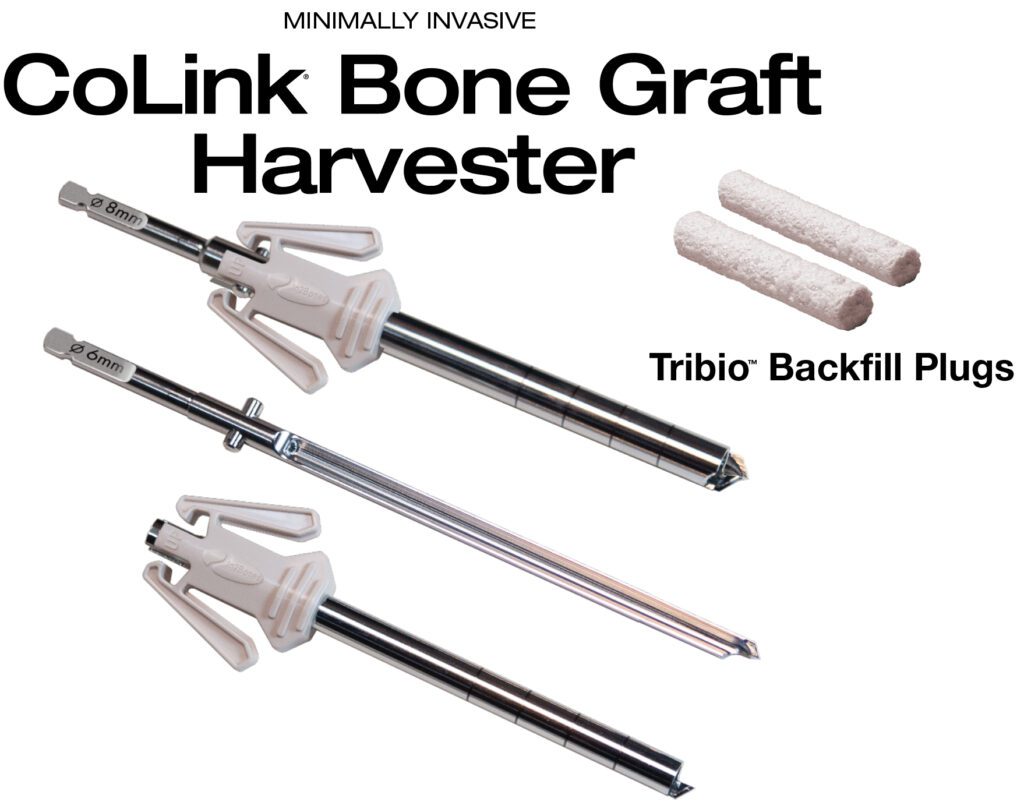
Read MoreIn2Bones Introduces CoLink Bone Graft Harvester And Tribio Backfill Plugs
In2Bones Global, Inc. today announced the U.S. commercial launch of its CoLink® Bone Graft Harvester and Tribio™ Backfill Plugs System. Packaged sterile and pre-assembled for single use, the CoLink Bone Graft Harvester is a minimally invasive bone graft device that harvests bone from various sites in the body, including the calcaneus, iliac crest, proximal tibia, distal tibia, and distal femur.…
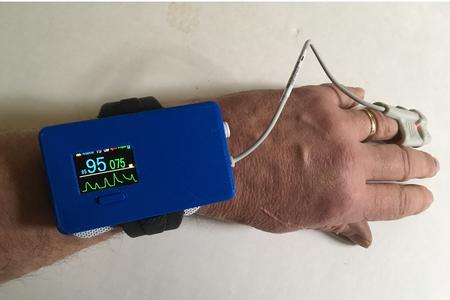
Read MoreMed-botics Wearable Device Detects Opioid Overdose, and can Provide Early Warnings to Over-sedated Patients
The FDA granted Breakthrough Device status to Med-botics, LLC, (www.med-botics.com), for its Oxalert EPOTM (Enhanced Pulse Oximeter) device. The wrist-worn arousal device is designed to prevent respiratory arrest and death from opioid overdose. FDA Guidance documents describe Breakthrough Devices as “innovative technologies that diagnose or treat life-threatening conditions more effectively than any FDA-approved products.” The FDA…
Read MoreAnaconda Biomed Announces First-in-Human Study of Next-Generation Thrombectomy System
Anaconda Biomed, a medical technology company developing a next-generation thrombectomy system for the treatment of ischemic stroke, has announced the completion of initial patient cases in a first-in-human study at Hospital Vall d’Hebron in Barcelona. This 125-patient, prospective, multi-center study will assess system safety and reperfusion measured using the modified treatment in cerebral infarction (mTICI) score. Study…
Read More