Diagnostics & Healthcare News
Grand Piano Passion Identifies Best Hearing Aid Technology for Music
Grand Piano Passion™, the leading resource for musicians and music lovers with hearing loss, reviews hearing aids and alternative devices for music in their new technology guide. Access the Guide: Hearing Aids and Music Technology. Half of classical musicians and one-third of rock musicians have hearing loss, according to a Hearing Research study, while music lovers…
Read MoreQuanta™ Receives FDA 510(k) Clearance for Expanded Indication of Continuous Renal Replacement Therapies
Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for an expanded indication of the Quanta Dialysis System, a compact and easy-to-use hemodialysis device, for two modalities of continuous renal replacement therapy (CRRT): continuous venovenous hemodialysis (CVVHD)…
Read MoreMagnus Medical Announces First Participants Treated in Study Using SAINT Neuromodulation System for Major Depression
Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for the treatment of neuropsychiatric disorders, today announced that the first participants have been treated in the Open Label Optimization (OLO) Clinical Trial evaluating the effectiveness of the Magnus Neuromodulation System with SAINT™ Technology for the treatment of Major Depressive Disorder (MDD).…
Read MoreFDA Clears ReddyPort® Non-Invasive Ventilation Device
ReddyPort announced today that the US Food and Drug Administration (FDA) granted 510(k) premarket clearance for the ReddyPort elbow device used in non-invasive ventilation (NIV). “ReddyPort’s patented elbow is central to the eco-system we are building to help mitigate clinical obstacles tied to NIV therapy from dry-mouth, oral biofilm accumulation to speech recognition,” says Tony Lair,…
Read MoreInvictus Announces FDA Clearance of Ground-Breaking Neoasis® ANC Device
Invictus Medical announced today that the culmination of their De Novo application to the FDA for its Neoasis® incubator-based active noise control (ANC) device has resulted in a clearance-for-use declaration by the FDA. “With this clearance for use, Invictus has made a huge step towards deploying the Neoasis® ANC device in neonatal intensive care units. It is well documented…
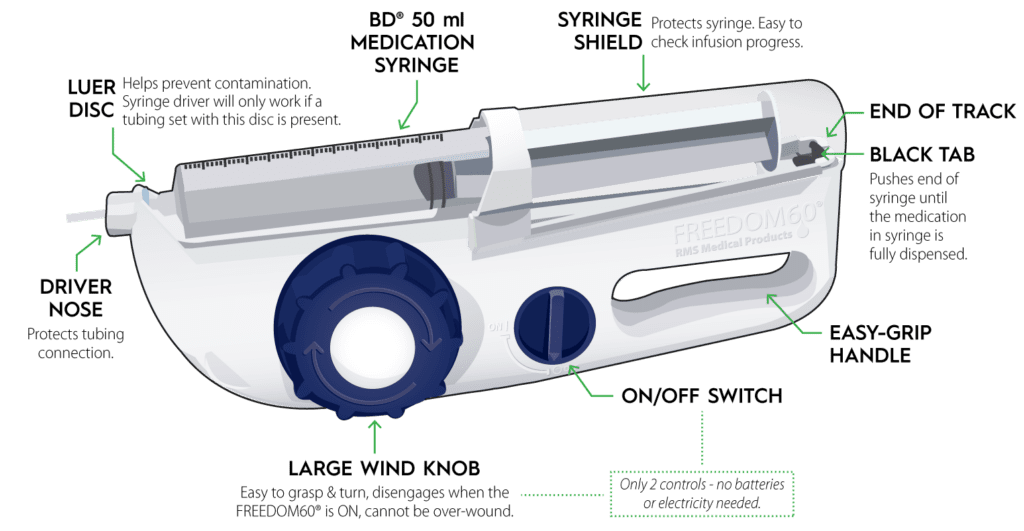
Read MoreKORU Medical Systems, Inc. Announces 510(K) Submission for Freedom60® Infusion System With Hizentra® 50 Ml Prefilled Syringes
KORU Medical Systems, Inc. (NASDAQ: KRMD) (“KORU Medical” or the “Company”), a leading medical technology company focused on the development, manufacturing, and commercialization of innovative and easy-to-use specialty subcutaneous infusion solutions that improve quality of life for patients, today announced that it has submitted a 510(k) premarket notification submission to the U.S. Food and Drug…
Read MoreNew FDA Clearance Makes Eyenuk the First Company with Multiple Cameras for Autonomous AI Detection of Diabetic Retinopathy
Eyenuk, a global artificial intelligence (AI) digital health company, and the leader in real-world applications for AI Eye Screening™ and AI Predictive Biomarkers™, has received U.S. Food and Drug Administration (FDA) clearance to use the Topcon NW400 retinal camera with its EyeArt AI system to automatically detect diabetic retinopathy (DR), adding to the already-cleared usage with Canon…
Read MoreOwlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
Owlet (NYSE: OWLT, “the Company”), the pioneer of smart baby monitoring, announces clearance from the U.S. Food and Drug Administration (“FDA”) of BabySat™, the first medical pulse-oximetry device featuring its advanced, wire-free sock design. Owlet is a leader in infant health data, having monitored more than 1 million babies, and with BabySat, combines its consumer-first…
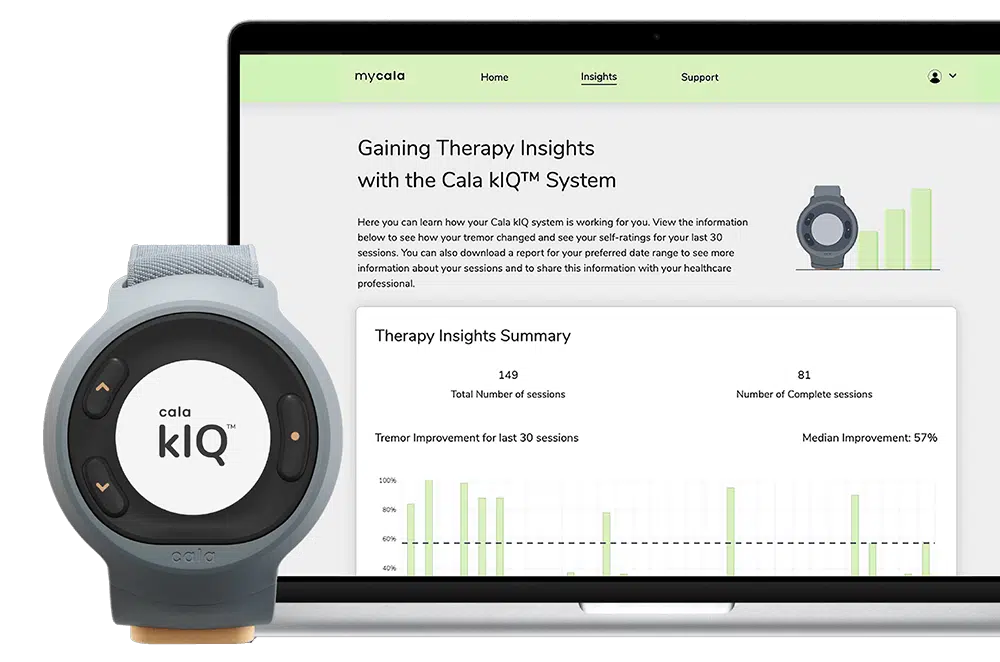
Read MoreCala® Launches The Cala kIQ™ System, Offering Meaningful Tremor Relief for Patients With Essential Tremor and Now Parkinson’s Disease
Cala, the bioelectronic medicine leader setting a new standard of care for chronic disease, today announced the commercial launch of its next generation system: the Cala kIQ™ System, the first and only FDA-cleared wearable device that delivers effective therapy for action hand tremor relief in people with essential tremor and Parkinson’s disease. The Cala kIQ…
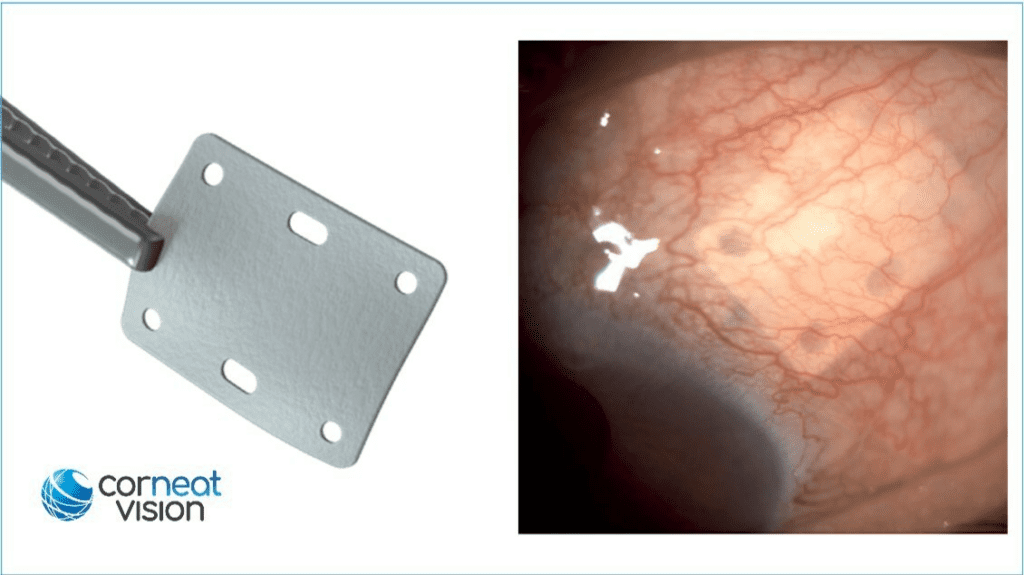
Read MoreFDA Clears CorNeat EverPatch, World’s First Non-degradable, Synthetic Tissue Substitute for Ophthalmic Surgery
CorNeat Vision’s EverPatch, a synthetic tissue substitute, has been granted 510(k) clearance by the US Food & Drug Administration (FDA). The CorNeat EverPatch is the first synthetic, non-degradable tissue-integrating matrix for use in ophthalmic surgeries. It is composed of a non-woven, polymer matrix which integrates with surrounding tissue and is intended to reinforce the sclera and…
Read More