Diagnostics & Healthcare News
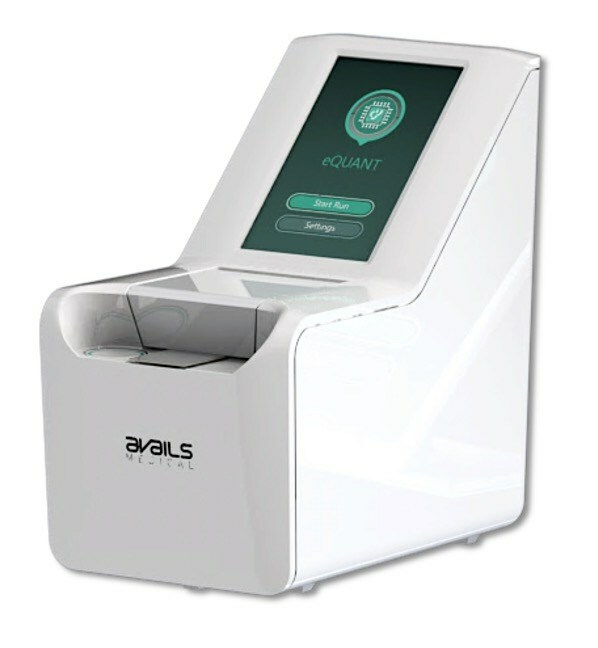
Avails Medical’s eQUANT™ System Submitted to FDA for 510(k) Clearance
Avails Medical, a pioneer in rapid, automated and fully electrical antibiotic susceptibility testing (AST) solutions announced today the submission of its eQUANTTM system for FDA 510(k) clearance. The eQUANT system provides a standardized inoculum (0.5 McFarland equivalent) directly from a positive blood culture, which is designed to be used with traditional automated AST systems and Disk…
Read MoreBiosency detects early COPD exacerbations thanks to Bora care®, a predictive digital medical device
The American Thoracic Society International Conference (ATS 2023) took place in Washington, D.C. from 19 to 24 May 2023. During the conference on 23 May, French MedTech Biosency unveiled its latest clinical results demonstrating the ability of its BVS3 technology to predict acute COPD (chronic obstructive pulmonary disease) exacerbations on average 3 days prior to hospitalisation in 86% of cases, with…
Read MoreiHealthScreen Inc. Announces FDA 510K Submission for iPredict™ AMD tool
iPredictTM AI Eye Screening System provides fully automated age-related macular degeneration (AMD) screening, including retinal imaging and immediate reporting of actionable results. Using the iPredictTM System, primary care and various specialty practices can accurately and efficiently screen people over 50 for AMD and refer them to a specialist (i.e., an ophthalmologist). Once high-resolution images of the patient’s eyes…
Read MoreBionix® Introduces IsoMark™ A Revolutionary Patient Marking Device for Radiation Oncology Treatment
For more than a decade, Bionix has supplied the radiation oncology sector of the healthcare industry with Accu-Tatt®, an all-inclusive, sterile, single-use tattooing device that marks targeted areas on a patient’s body for proper alignment during radiation therapy procedures. Working with an inventive medical professional with years of experience applying tattoos to patients to prepare…
Read MoreSibel Health Announces a New FDA-Clearance for Advanced Wireless Monitoring in Neonates and Infants
Sibel Health, an award-winning digital health company spun out of the Querrey Simpson Institute for Bioelectronics at Northwestern University, announces a new 510(k) clearance from the U.S. Food and Drug Administration (FDA) for continuous neonatal and infant monitoring for babies born of any gestational age to infants of 2 years at the International Maternal Newborn Health Conference in Cape…
Read MoreClinical Study Results of the BlueWind System for Patients with Overactive Bladder Featured at the 2023 AUA Annual Meeting
BlueWind Medical, Ltd., the developer of a transformative implantable tibial neuromodulation therapy for Overactive Bladder (OAB), today announced results from the OASIS pivotal trial evaluating the safety and efficacy of the BlueWind System in the treatment of OAB. The results were featured in the late-breaking session (LBA01-05) at the American Urological Association (AUA) 2023 Annual Meeting…
Read MoreAd Astra Diagnostics Files 510(k) for QScout™ Hematology Analyzer
Ad Astra Diagnostics (AAD), developer of rapid diagnostic systems, announced that it has submitted a 510(k) application to the U.S. Food and Drug Administration (FDA) for its QScout RLD rapid-result hematology system intended to report white blood cell count, neutrophil-to-lymphocyte ratio, and counts of six white blood cell types in fingerstick or venous blood. QScout is the…
Read MoreNanoVibronix Announces Positive Results from Independent Product Trial of UroShield for Patients with a Spinal Cord Injury
NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company’s proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced the positive evaluation results for its UroShield device, presented at a recent medical conference by clinicians from the Royal National Orthopaedic Hospital (“RNOH”). The report concluded that the Company’s UroShield…
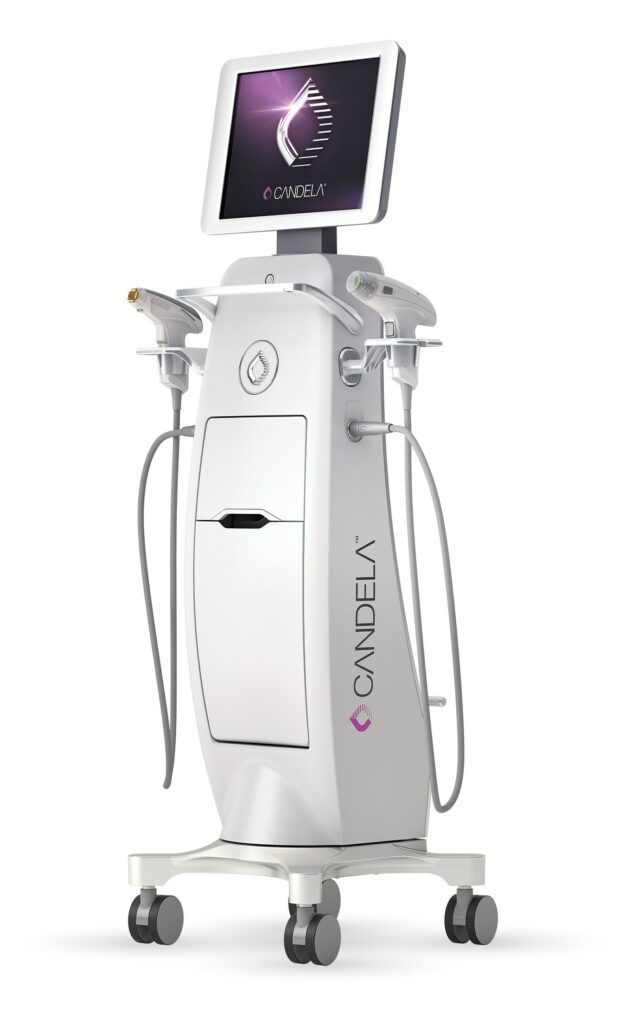
Read MoreCandela Introduces the New FDA-Cleared Profound Matrix™ System
Candela, the leading provider of energy-based solutions worldwide, and the pioneer of the first and only long-pulse radiofrequency (RF) microneedling device, announces the launch of the all-new Profound Matrix™ system. Designed to correct, maintain, and restore skin at various stages of the aging journey, this multi-application system features the Sublime™, Sublative™ RF, and all-new Matrix…
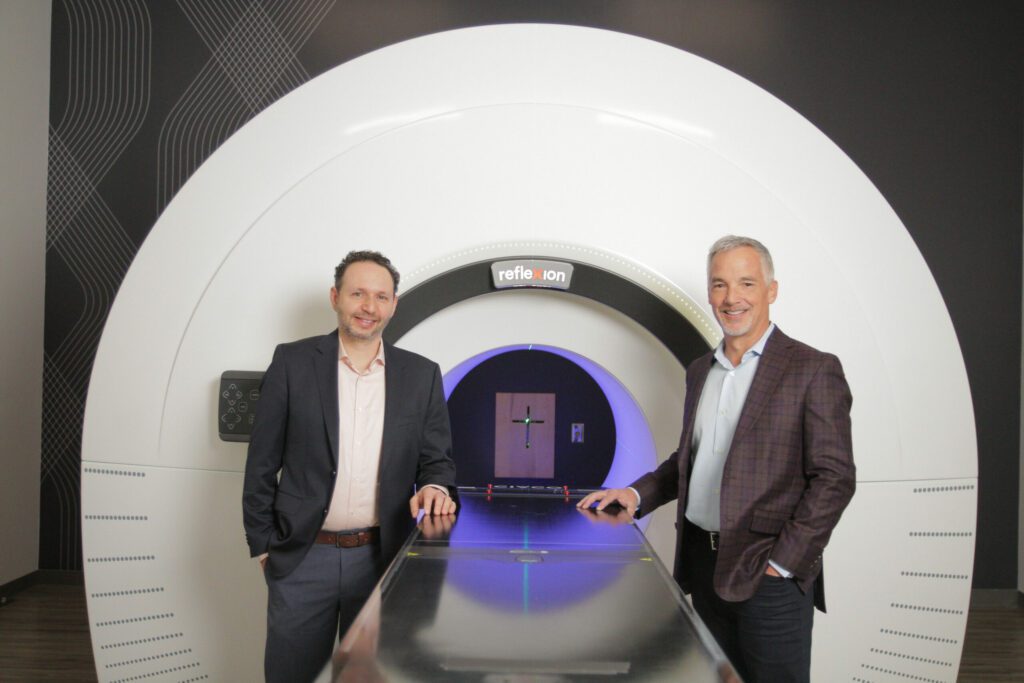
Read MoreRefleXion Receives FDA Clearance for SCINTIX Biology-Guided Radiotherapy; Cutting-edge Treatment Applicable for Early and Late-stage Cancers
RefleXion Medical, a therapeutic oncology company, announced the U.S. Food and Drug Administration (FDA) has granted the first marketing clearance for its SCINTIX™ biology-guided radiotherapy, a cutting-edge treatment applicable for early and late-stage cancers. The company will host a live-stream event featuring both its co-founder and CEO discussing the breakthrough significance of SCINTIX therapy for cancer…
Read More